Antibiotic resistance can occur when infected patients misuse prescribed antibiotics, either by not completing their course of the drugs or taking them when unnecessary. Improper use of antibiotics can result in many mechanism changes, including the bacteria evolving and changing their structure to prevent the drugs from infiltrating the infection, thus making infections nearly impossible to treat. Antibiotic resistance is deeply alarming, as not only does it heavily increase the risk of sepsis but it also has a domino effect on public health as it increases the risk of routine surgical procedures and immunotherapy. So much so that the World Health Organization (WHO) has declared antibiotic resistance as the biggest global public health threat, with 1.25 million deaths in 2019 alone.
For instance, if an individual were to contract MRSA (methicillin-resistant Staphylococcus aureus) (also known as the ”Superbug”), it would be extremely difficult to treat due to its resistance and could therefore be fatal. Vancomycin is one of the few antibiotics that can effectively treat MRSA, and this has been the case for the past 66 years; however, some may argue that it’s time to research and test additional treatments as Vancomycin still isn’t a perfect drug. This is because, as expected, it has side effects that include nephrotoxicity, which is the rapid degeneration of the kidneys as a result of medication/chemicals. This can prove to be problematic in the treatment of the elderly, especially as those of old age are more susceptible to bacterial infections.
In the same breath, however, the trials currently being conducted have the potential to trigger a paradigm shift in medicine. For instance, a news article at GlobalData recently discussed a clinical trial being sponsored by Pfizer (with AstraZeneca as collaborators), titled ‘To Investigate the Safety and Tolerability of Aztreonam-Avibactam (ATM-AVI)’. The trial is centred around the potential use of a new antibiotic for treating infections that have become multidrug-resistant. In summary, the article detailed that the EMA is advocating for Emblaveo, which is a combination of Aztreonam and Avibactam, to treat other bacterial infections that may be resistant to their first line of therapy (not including MRSA). The new antibiotic would work by binding to the proteins on the surface of the bacteria and ultimately killing them. The second half of Emblaveo, Avibactam, is known to block beta-lactamases, and these enzymes are essentially responsible for making certain bacteria resistant to antibiotics, but blocking them prevents that.
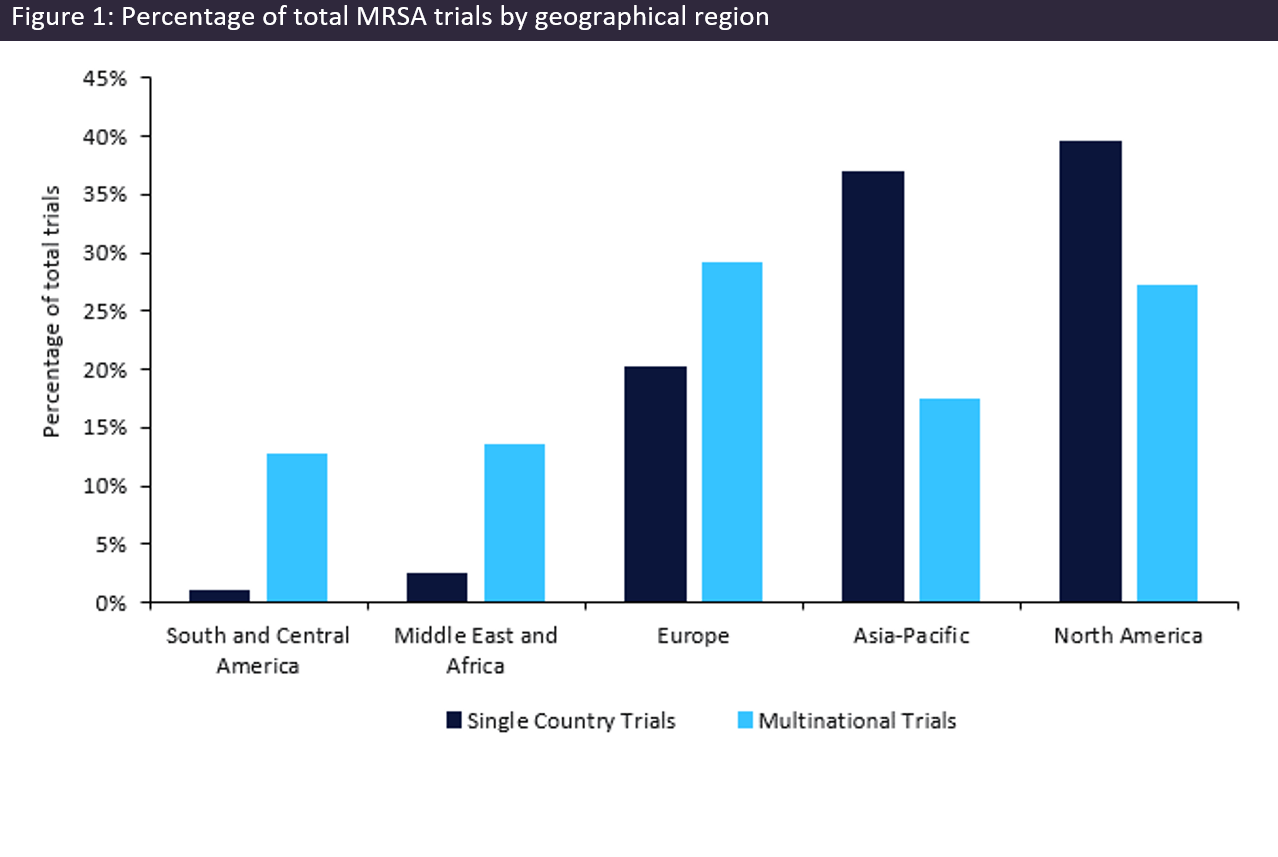
MRSA is a bacteria that isn’t particularly uncommon because of its prevalence in hospital settings. MRSA prevalence is particularly high in Asia, making it the epicentre for resistance. This is most likely because of the population taking antibiotics in excess. This is highlighted in the GlobalData Clinical trials database, where 36.85% of the single-country trials took place in Asia-Pacific, with 36.88% of those trials taking place in China. Overall, most trials were conducted in North America (see figure 1, above). By observing this data, it’s clear that not enough trials are being conducted even though antibiotic resistance has been declared “the biggest global health threat”.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData