Before the use of antibiotics became widespread throughout the 1940s, 43% of people died from infections such as tuberculosis, pneumonia and even strep throat; 90% of children with bacterial meningitis died and those who lived had severe life-altering disabilities. It is difficult to imagine returning to these times, coined the “dark ages” almost a decade ago by the then-prime minister of the UK, David Cameron.
However, young children continue to be at significant risk: in 2019, one in five deaths caused by antimicrobial resistance (AMR) occurred in children under the age of five. The global burden of AMR is more urgent now than ever before, as the United Nations predicts annual worldwide deaths due to drug-resistant bacteria will exceed 10 million by 2050.
AMR occurs when disease-causing microorganisms evolve mechanisms that protect them against the effects of anti-infective therapies. Although AMR occurs naturally over time, the misuse and overuse of antimicrobials are the major drivers accelerating the emergence and spread of AMR. Other factors, such as a lack of clean water, sanitation and adequate infection prevention, promote the spread of resistant microbes. These issues primarily affect low and middle-income countries, which are in turn disproportionally affected by drug-resistant infections.
However, AMR is undoubtedly a global health challenge. Research conducted as part of the Global Research on Antimicrobial Resistance project published in The Lancet revealed that 2.8 million antimicrobial-resistant infections occur each year in the US alone. They also discovered that 4.95 million deaths per year were associated with AMR, leading to their urgent call for action.
Although the World Health Organization described AMR as one of the top 10 global threats to public health in 2019, the clinical pipeline of new antimicrobials remains stagnant. This was corroborated by data obtained from GlobalData’s Clinical Trials Intelligence database, which showed anti-infective clinical trials have plateaued over the last 10 years, which is why many consider it a silent pandemic. The brief uptake in 2020 aligns with the increased investment in antimicrobial resistance research throughout the Covid-19 pandemic.
However, the proportion of anti-infective clinical trials out of total clinical trials per year has declined (Figure 1), showing investment in clinical trials appears to have reached a 10-year low. So, it is no surprise that no new classes of antibiotics have been discovered since the 1980s.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData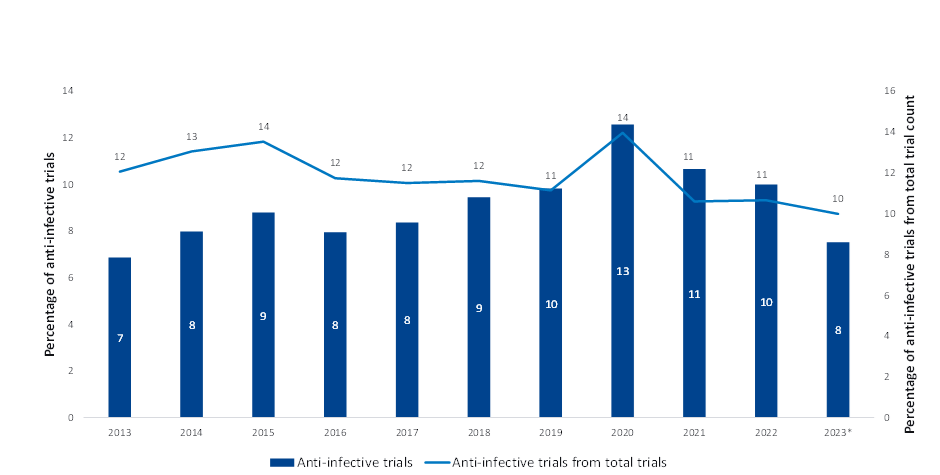
| Note * Date through October 2023 Source: GlobalData, Pharma Intelligence Center (Accessed October 2023) ©GlobalData |
Although the quantity of AMR research does not reflect its significant threat to public health, several entities are actively engaged in addressing the challenges of AMR and are investing in the development of new antimicrobial treatments and diagnostic tools. The largest ever investment in global AMR surveillance by any country was announced in August and comes from the UK government’s aid budget, which will invest $255m to reduce the burden of AMR across Asia and Africa over the next three years.
The US also launched its $104m grant for drug-resistant bacteria research in September. Several pharmaceutical companies are also recognising this issue, such as INTEGRA Biosciences, which is providing its pipetting solutions to speed up molecular diagnostics for AMR testing, as well as GSK, which has multiple R&D projects targeting priority pathogens.
There are also numerous start-ups involved in the movement. Xingji Biological raised $14.6m earlier this year, which will be used for its Phase I clinical trial for its anti-infective macromolecular drug targeting anti-methicillin-resistant staphylococcus aureus. Similarly, BioVersy raised an additional $9m at the start of the year to support the clinical development of BV100, a potential breakthrough antibiotic targeting the most drug-resistant bacterial pathogen, Acinetobacter baumannii.
Some argue that science is playing catch-up in its efforts to mitigate the continued devastation of AMR and insights from GlobalData’s Clinical Trials Intelligence platform indicate these efforts may be too little, too late. However, it is clear that there is a concerted effort to address the global challenges of AMR.
Alleviating AMR requires a multifaceted approach incorporating improved education, innovative surveillance measures and the development of new treatment options and diagnostic tools. It is also imperative that the efficacy of existing antimicrobials be preserved. Global collaboration and awareness will be key to addressing the challenges posed by AMR to global health.