According to leading data and analytics company GlobalData’s Clinical Trials Database, among the clinical trials for 2023, Phase II led with 41.0% of trials.
This was followed by Phase I (26.6%), Phase III (17.0%), and Phase IV (15.4%).
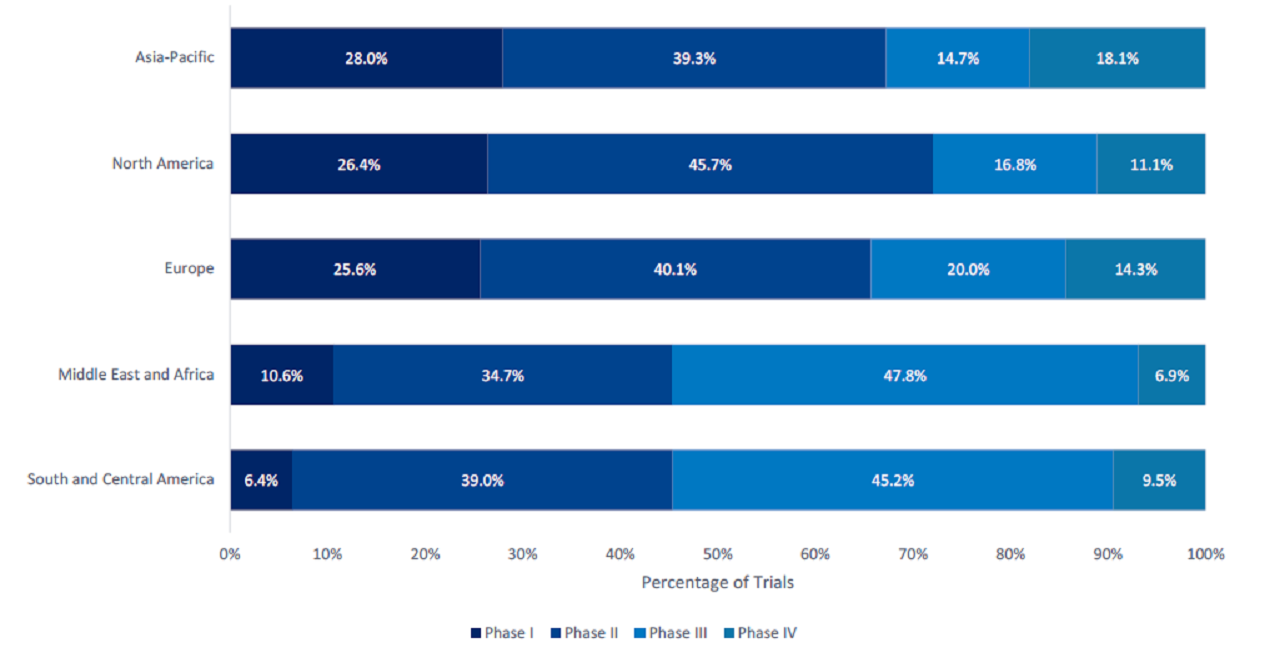
Out of all five researched regions, Asia-Pacific (APAC) had the greatest proportion of early-stage, Phase I trials at 28.0% (Figure 1). Overall, the majority of APAC trials were in Phase II (39.3%).
North America had the highest proportion (45.7%) of Phase II trials. The Middle East and Africa had the largest proportion (47.8%) of Phase III trials, followed by South and Central America (45.2%). APAC had the highest proportion (18.1%) of Phase IV trials, followed closely by Europe (14.3%).
Phase I trials accounted for 39.2% of trials in China, compared to 40.7% in 2022.
Phase II dominated trials in the US with 45.7%. Iran led Phase III trials with more than 62.8%.
Most of these trials investigated the central nervous system, followed by women’s health and infectious diseases.
Iran was one of the few Middle Eastern nations with the capacity to develop therapeutics and vaccines.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataIndia had the highest proportion (25.3%) of trials in Phase IV, followed by China at 16.3%.
Similar to previous years, this could be attributed to India’s rule that drugs approved in the UK, EU, Australia, Canada, Japan, or the US no longer need clinical trials in the country, which sped up drug approvals and removed large study requirements.
However, pharmaceutical companies still need to conduct Phase IV trials after the drug has been marketed to gather post-marketing, real-world evidence and evaluate its long-term effects.