Cannabinoids are a group of substances found in the cannabis plant. More than 100 cannabinoids have been identified, with tetrahydrocannabinol (THC) and cannabidiol (CBD) being the central components. Countries such as Australia, Canada, most countries in South America and most of the US have legalised the usage of medicinal cannabinoids for the treatment of numerous conditions, such as seizures and pain. Many of the key takeaways in a December 2020 GlobalData report are the same today, such as the top sponsor and the top indication.
GW Pharmaceuticals continues to be the top industry sponsor for cannabinoid clinical trials, with its trials greatly outnumbering those from other sponsors. The company currently has 99 trials, 15 fewer than what was previously reported (114). Many of these trials are now under Jazz Pharmaceuticals, as it acquired GW Pharmaceuticals in February last year. GW Pharmaceuticals’ main drug candidates include Epidiolex and Sativex. Epidiolex is an oral solution that is a competitive antagonist at the G-protein coupled receptor, GPR55, inhibiting the binding of a membrane phospholipid, lysophosphatidylinositol (LPI). Sativex is a mouth spray composed of two principal cannabinoid components, CBD and delta-9 tetrahydrocannabinol. Other top industry sponsors include Sanofi and Insys Therapeutics.
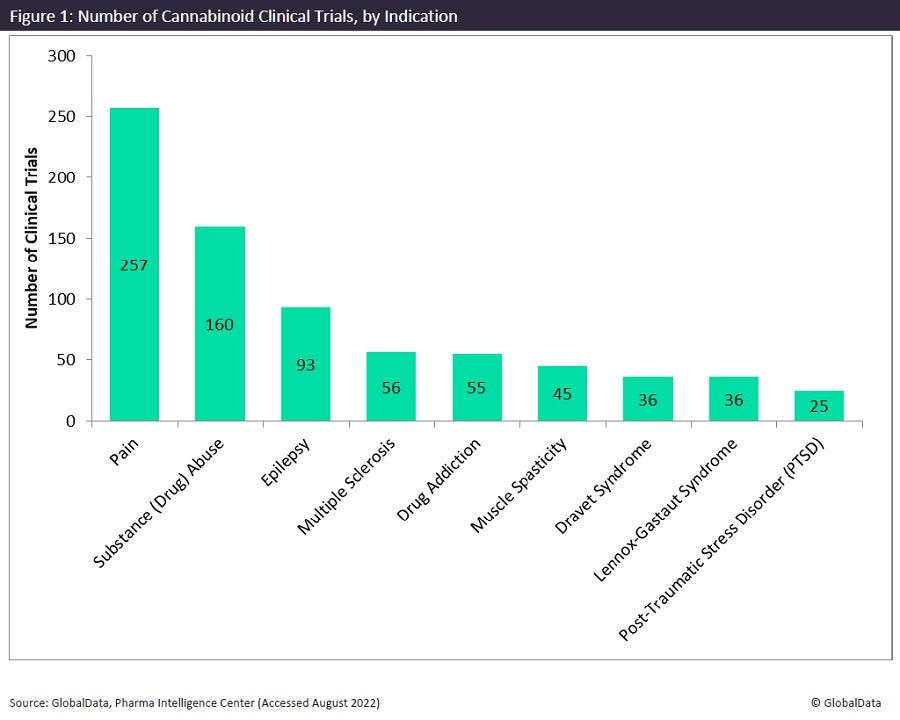
As for indication, pain is at the top of the list, the same as the December 2020 report. There are 257 cannabinoid trials testing the treatment of pain. This was followed by substance (drug) abuse, epilepsy, multiple sclerosis, and drug addiction (Figure 1).