At the recent European Alliance of Associations for Rheumatology (EULAR) 2024 conference, chimeric antigen receptor (CAR) T-cell modalities in autoimmune diseases, including systemic lupus erythematosus (SLE) and lupus nephritis (LN), was featured as a key topic.
This modality’s potential to achieve drug-free remission in patients with autoimmune diseases such as SLE and LN is a key focus that pharma companies continue to explore.
The results presented at EULAR 2024 from ongoing, industry-sponsored, early-stage clinical trials generally gave early indications that CAR T-cell assets may be able to achieve an immune reset and potentially help patients achieve drug-free remission.
However, reports of relapse post-infusion have drawn cautious optimism about an otherwise promising display of results for CAR T-cell assets in this disease space.
Achieving drug-free remission would represent a transformative, paradigm-shifting moment in the care of patients with SLE and LN.
Results from ongoing clinical trials indicate a movement in the right direction for CAR T-cell assets in SLE and LN.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataKyverna Therapeutics’ KYV-101 exhibited disease control at a follow-up period, with six of the seven patients with LN not requiring immunosuppressants and two of these patients having completed at least six months post-infusion.
Results from Cabaletta Bio’s CABA-201 RESET-SLE trial indicated an improvement in the SLE Disease Activity Index 2000 score and resolution of vasculitis, arthritis, and haematuria within four weeks post-infusion in one patient with SLE, with ongoing taper from prednisone 10mg per day.
Preliminary data from JW Therapeutics’ JWCAR029 also suggested efficacy, with three patients in the low-dose group showing a reduction in Safety of Estrogens in Lupus Erythematosus National Assessment–SLEDAI scores at four months follow-up.
Additionally, all three patients achieved SLE responder index-4 while two patients reached lupus low disease activity status.
iCell Gene Therapeutics’ BCMA CD19 compound CAR T cells also delivered promising results that demonstrated SLE patients free of lupus medication at a mean follow-up period of 20 months and in LN patients at a mean follow-up period of 16 months.
While the general sentiment over these results has been positive, episodes of relapses among patients have generated caution, including in the case of one patient who received KYV-101 and relapsed five months post-infusion.
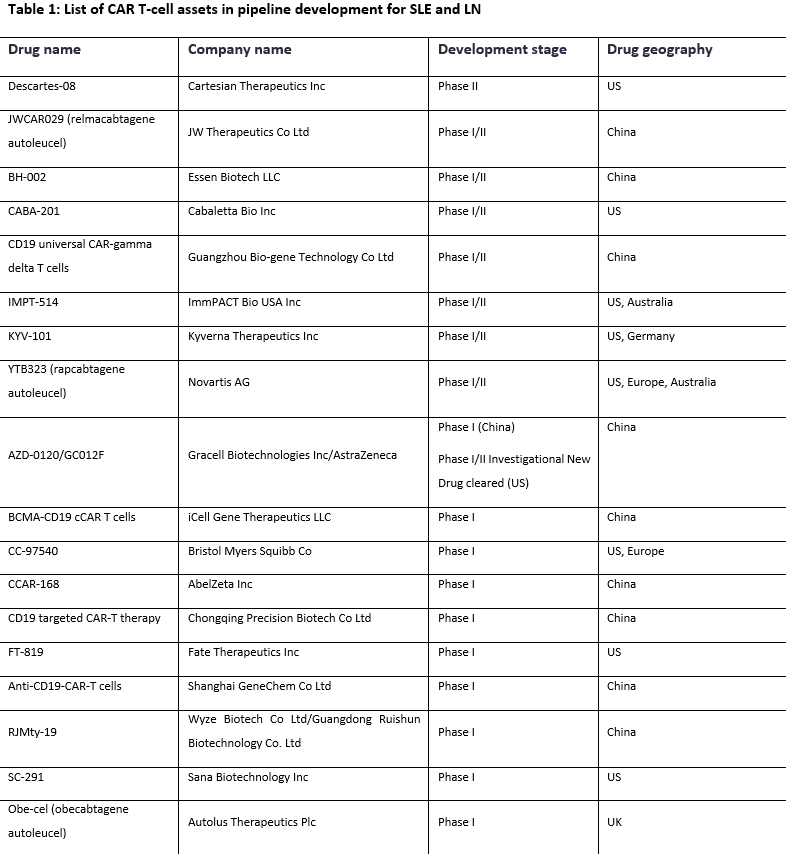
According to leading data and analytics company GlobalData’s Pharmaceutical Intelligence Center, of the 18 CAR T-cell assets currently in pipeline clinical development for SLE/LN, 50% of pipeline assets are being evaluated in China (Table 1).
This trend indicates the emergence of China as a leader in the development of CAR T-cell therapies within the autoimmune disease space.
The trend also highlights the opportunity for large pharma companies to partner with companies conducting clinical trials in China to propel the growth of the CAR T-cell modality on a broader geographical scale.
Clinical developments and commercial partnerships within the CAR T-cell space will be crucial to meet the unmet needs that exist within the SLE and LN disease spaces.
Key opinion leaders interviewed by GlobalData have emphasised the need for agents that can help patients, especially those with refractory forms of the disease, into remission without the need for these patients to be on traditional steroid and immune-suppressive therapies.
As these innovator modalities continue along the path of development, clinical milestones achieved during this process will be seen as key moments that will propel the growth of this market.
| Table 1: List of CAR T-cell assets in pipeline development for SLE and LN | |||
| Drug name | Company name | Development stage | Drug geography |
| Descartes-08 | Cartesian Therapeutics Inc | Phase II | US |
| JWCAR029 (relmacabtagene autoleucel) | JW Therapeutics Co Ltd | Phase I/II | China |
| BH-002 | Essen Biotech LLC | Phase I/II | China |
| CABA-201 | Cabaletta Bio Inc | Phase I/II | US |
| CD19 universal CAR-gamma delta T cells | Guangzhou Bio-gene Technology Co Ltd | Phase I/II | China |
| IMPT-514 | ImmPACT Bio USA Inc | Phase I/II | US, Australia |
| KYV-101 | Kyverna Therapeutics Inc | Phase I/II | US, Germany |
| YTB323 (rapcabtagene autoleucel) | Novartis AG | Phase I/II | US, Europe, Australia |
| AZD-0120/GC012F | Gracell Biotechnologies Inc/AstraZeneca | Phase I (China) Phase I/II Investigational New Drug cleared (US) | China |
| BCMA-CD19 cCAR T cells | iCell Gene Therapeutics LLC | Phase I | China |
| CC-97540 | Bristol Myers Squibb Co | Phase I | US, Europe |
| CCAR-168 | AbelZeta Inc | Phase I | China |
| CD19 targeted CAR-T therapy | Chongqing Precision Biotech Co Ltd | Phase I | China |
| FT-819 | Fate Therapeutics Inc | Phase I | US |
| Anti-CD19-CAR-T cells | Shanghai GeneChem Co Ltd | Phase I | China |
| RJMty-19 | Wyze Biotech Co Ltd/Guangdong Ruishun Biotechnology Co. Ltd | Phase I | China |
| SC-291 | Sana Biotechnology Inc | Phase I | US |
| Obe-cel (obecabtagene autoleucel) | Autolus Therapeutics Plc | Phase I | UK |