Cell and gene therapies (CGTs) have been gaining traction within the medical community as treatment options that are expected to replace existing lifelong treatments for the management of chronic diseases.
Specifically, CGTs should require fewer doses than current long-term treatments, which could offer significant benefits for both patients and strained healthcare systems.
Over the past decade, a total of 14 CGTs were approved across various therapy areas in the US.
According to leading data and analytics company GlobalData’s Drugs Database, of these 14 therapies that were approved by the US Food and Drug Administration (FDA), more than 70% were approved within the last five years (2019-2023).
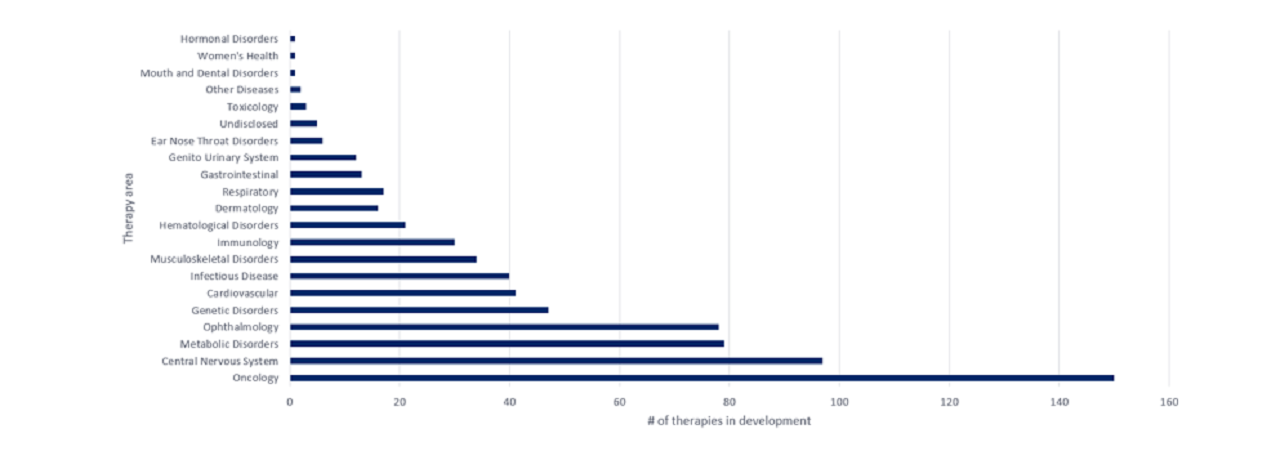
The opportunity for the approval of more CGTs remains high. According to GlobalData’s Drugs Database, there were 694 CGTs in development across various therapy areas in the US as of February 2024.
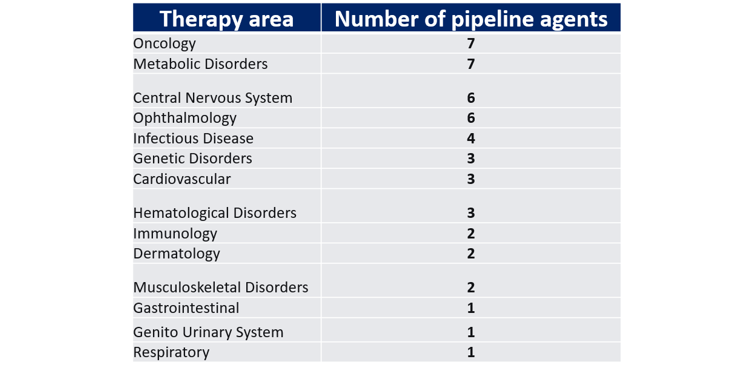
Of these 694 CGTs, 48 assets are in late-stage development. Specifically, five assets are in preregistration and 43 assets are in Phase III development.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataPreviously, CGTs were viewed as promising solutions for patients with serious diseases, rare diseases, or illnesses with no available treatment options.
Now, the distribution of CGTs in late-stage development is more prevalent across several therapy areas, including cardiology, metabolic disorders, and central nervous system indications.
However, despite the robust CGT pipeline in the US, the degree to which scientific advancements in CGTs will translate into comparable progress in the US healthcare system remains uncertain, as the adoption of CGTs may be hampered by several obstacles such as concerns about clinical trials, regulatory obstacles, affordability, and access.
In the development of CGTs, challenges often arise early in the drug development process, particularly as companies advance pipeline assets to the clinical development stage where patient access and recruitment pose significant obstacles for clinical trials (NMDP, 2024).
This can be attributed to two main factors: the rarity of some of the indications being studied, which results in a small patient population that makes it difficult to achieve a large sample size, and the perception that CGTs are treatments of last resort, which dissuades patients with advanced disease from participating or remaining in trials.
Furthermore, even if companies successfully complete their clinical trial programmes and submit filings to the FDA, the regulatory landscape for CGTs may not be adequately prepared to keep pace with the rapid advancements in the field.
While the establishment of the Office of Therapeutic Products at the FDA’s Center for Biologics Evaluation and Research in March 2023 was a step towards addressing the anticipated increase in cell and gene therapy submissions, it remains unclear how the current regulatory framework will be adapted to effectively address the unique challenges introduced by CGTs (FDA, 2023).
The high costs associated with CGTs pose a significant barrier to their adoption in the US, despite the potential therapeutic benefits they offer.
The substantial production costs, particularly for gene therapy applications that require high vector doses such as those targeting skeletal muscle, contribute to these high price points (Cornetta et al, 2022).
In turn, this presents challenges to accessibility, especially within Medicaid’s current payment systems.
However, there is potential for overcoming this challenge with the introduction of bipartisan legislation such as the Medicaid Value-Based Purchasings for Patients Act (H.R. 2666), which aims to improve Medicaid patient access to new, effective, and high-cost therapies through value-based purchasing arrangements (US Library of Congress, 2023).
Cell and gene therapies are reshaping medicine by offering personalised treatments tailored to individual genetic and cellular profiles.
This approach shows promise for addressing previously challenging diseases, potentially reducing reliance on conventional treatments and improving patient outcomes.
Additionally, the regenerative potential of some therapies offers hope for conditions such as spinal cord injuries and neurodegenerative disorders.
While initial costs may be a consideration, the long-term benefits include reduced healthcare expenditures.
Ongoing research in this field holds the potential to uncover new applications and refine existing approaches, further expanding therapeutic options for patients.