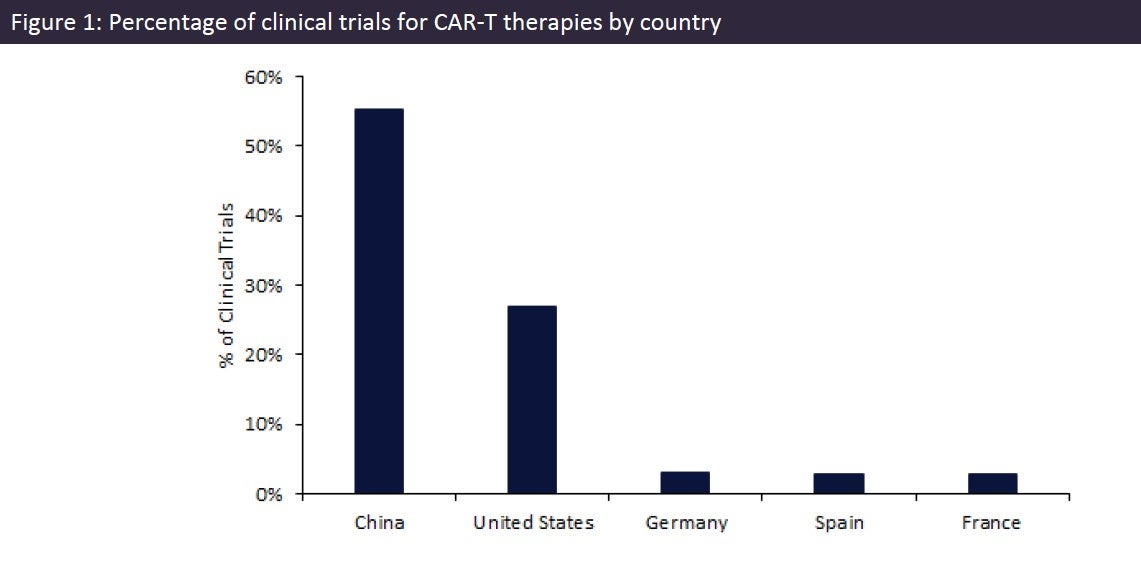
Chimeric antigen receptor (CAR)-T cell therapy is an innovative approach within cancer treatment. By genetically modifying a patient’s own immune cells to kill cancer cells, CAR-T therapies have shown promising results, especially for certain types of blood cancers. The efficacy of CAR-T therapies can vary depending on the type of cancer being treated and other factors such as patient characteristics and disease stage. According to GlobalData’s Clinical Trials Database, 48.1% of CAR-T trials are currently ongoing and recruiting, 20.7% are completed and 9.6% are planned. Currently, China is in the lead with the highest percentage of CAR-T trials at 55.3%, followed by the US at 26.9%, Germany at 3.1%, Spain at 3.0%, and France at 2.9%.
The reason for the high number of trials in China could be due to government initiatives to ensure new treatments come to market. The development of CAR-T therapies in China appears to outpace research happening in the West. Moreover, for each CAR-T therapy developed by US companies, there are 1.5 times more CAR-T therapies being investigated by Chinese companies. As part of Healthy China 2030 and other pharma and healthcare visionary programmes, China enhanced its focus on innovative therapies. China’s race for domination in this field means that of the ten companies involved in the development of CAR-T drugs, six are based in China. On average, the number of newly identified CAR-T therapies from Chinese developers has doubled every year since 2014.