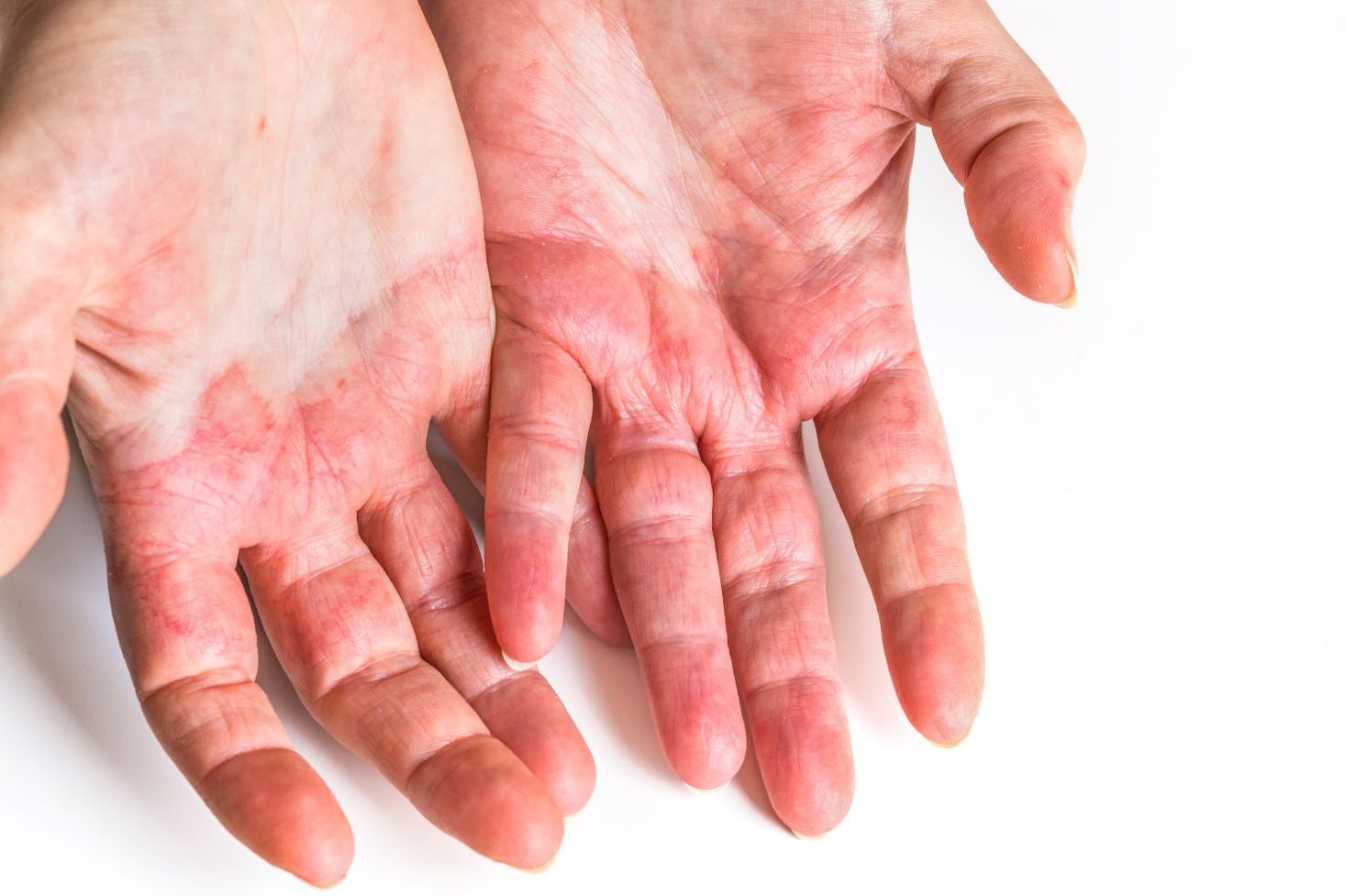
Chronic hand eczema (CHE) is a persistent, inflammatory skin disorder characterised by redness, itching, and the formation of vesicles on the hands that can result in pain and a significant reduction in quality of life (QoL). CHE is defined by its persistence (longer than three months) or its recurrences (at least two relapses per year after its initial manifestation and complete clearance between relapses) (Weisshaar, 2024). CHE is multifactorial, with genetics and the environment contributing to its development.
Chronic exposure to irritants, allergens, and repetitive hand washing are established risk factors for CHE, and individuals with a history of atopic dermatitis (AD) are also susceptible to developing CHE. Although multiple treatments for CHE are available, significant unmet need remains in long-term disease management and symptom control. Many patients do not experience complete relief from currently available therapies and suffer from frequent relapses. Managing CHE is challenging, as patients often require continuous treatment to maintain disease control, which can lead to issues such as skin atrophy and resistance to therapy (Leon, Berbegal and Silvestre, 2015). Therefore, there is a clear demand for therapies that offer sustained relief, fewer side effects, and improved ease of use.

Access deeper industry intelligence
Experience unmatched clarity with a single platform that combines unique data, AI, and human expertise.
Topical corticosteroids are frequently used as a CHE treatment and provide effective anti-inflammatory action. However, their long-term use is limited by their potential side effects, including skin thinning and the development of contact allergies. Emollients and moisturisers play a supportive role in symptom relief by hydrating the skin and repairing the skin barrier, but despite being beneficial, they do not address the underlying inflammation. Immunosuppressants such as calcineurin inhibitors offer an alternative to corticosteroids, particularly for patients with corticosteroid-resistant CHE, but their long-term use can be associated with neurotoxicity, renal dysfunction, and the development of metabolic syndromes including diabetes and hypertension (De Leon, Berbegal and Silvestre, 2015; Ruiz and Kirk, 2015).
GSK’s Toctino (alitretinoin) is the only therapy approved for CHE and has previously demonstrated good efficacy in eczema clearance (Blair and Scott, 2016). Nevertheless, research investigating new therapeutic agents, including biologics and small molecules targeting specific inflammatory pathways, may provide better alternatives to the current standard of care for CHE. Such agents have the potential to provide targeted treatment with improved efficacy and safety profiles, including LEO Pharma’s Anzupgo (delgocitinib), which has been approved in Japan for AD and is currently in clinical trials for CHE (Phase III) and AD globally. Head-to-head studies between Toctino and Anzupgo (NCT05259722), conducted by LEO Pharma, further demonstrated Anzupgo’s superiority in causing a reduction in the Hand Eczema Severity Index (HECSI) score from baseline to Week 12 compared to Toctino capsules as its primary outcome measure. Anzupgo also exhibited superiority in all key secondary outcome measures, including Investigator’s Global Assessment (IGA)-CHE treatment success, health-related QoL, and having a lower number of treatment-emergent adverse events (Leo Pharma, press release, 24 January 2024). Other clinical trials for CHE that include agents such as Sanofi/Regneron’s dupilumab (NCT04512339), Asana BioSciences’ gusacitinib (NCT03728504), and Afecta Pharmaceuticals’ AFX5931 (NCT03703895) have been completed and have shown positive responses against CHE.
Overall, current treatment options for CHE such as topical corticosteroids, emollients, immunosuppressants, and phototherapy may improve the condition, but all have limitations. Emerging research into the pathophysiology of CHE may contribute to the investigation of novel therapies that may offer better disease control with fewer side effects. Nevertheless, to address the unmet needs in CHE treatment, further research and clinical trials that focus on safety and the patient’s QoL are essential.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData