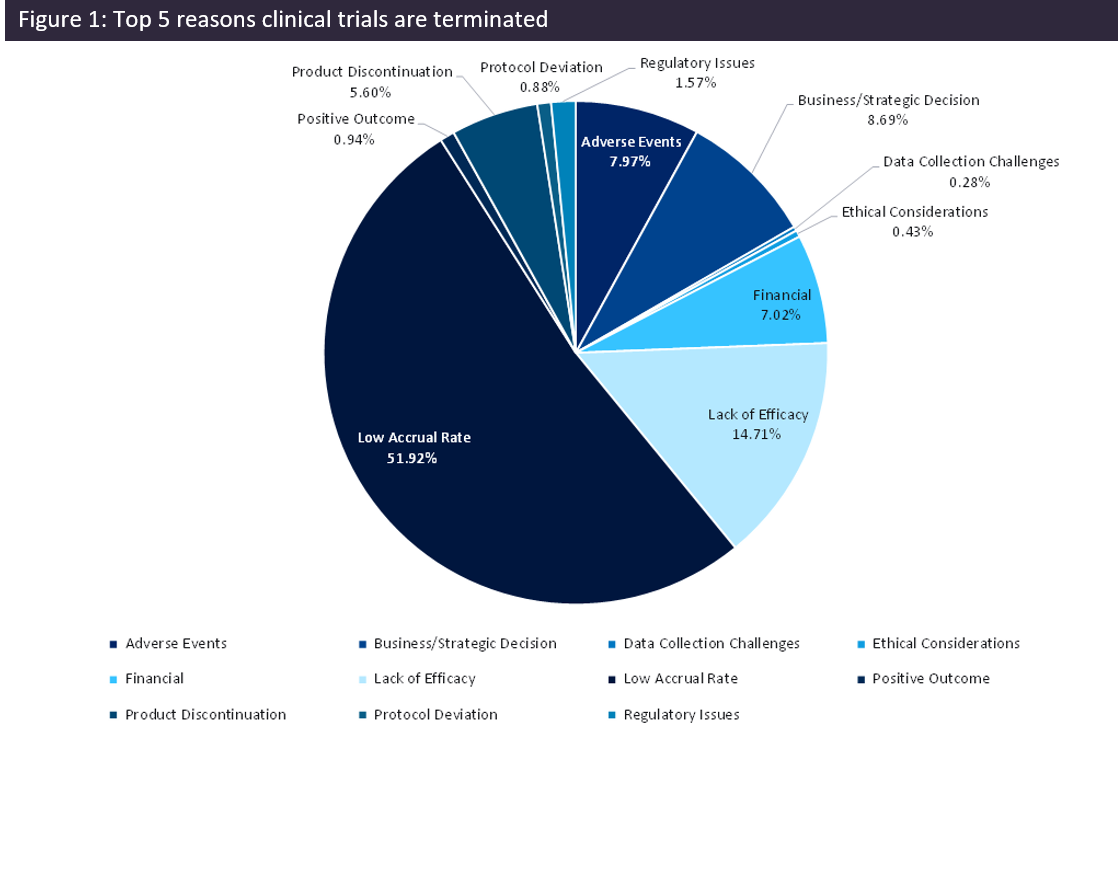
Data obtained from GlobalData’s trials intelligence platform corroborate the widely known fact that the leading reason clinical trials are terminated is due to low accrual (Figure 1). This is a large part of the reason that only around 10% of clinical trials result in an approved drug. There has been a spotlight on improving patient recruitment and retention as the industry recognises the value of patient-centricity and has begun shifting towards incorporating patient-centric approaches throughout clinical trials. In turn, this has also resulted in a lot of conversation around the performance of clinical trial sites and particularly, their efforts to meet trial recruitment targets.
One of the major topics at Clinical Trials Arena’s Outsourcing in Clinical Trials and Clinical Trials in Oncology event series throughout H1 has been case studies from reputable industry leaders emphasising the importance of meticulously screening trial sites to ensure you are securing the utmost quality. An insightful presentation delivered at Clinical Trials Oncology West Coast on 23-24 April, by James Eamma, executive director of therapeutic strategy, oncology at Worldwide Clinical Trials, discussed the ‘80/20’ rule, which says that 20% of your clinical trial sites will be doing 80% of the work. This means that selecting the right trial sites to work with is crucial for the success of your trial.
Correct site selection is undoubtedly fundamental and there is an abundance of conversation around the behavior of trial sites and the perceived lack of effort some sponsors experience. However, insight into the underrepresented perspective of a clinical trial site was shared by Suzy Montanye, site relationship manager, Endo Pharmaceuticals, this year at the Outsourcing in Clinical Trials East Coast May 20–21 conference. She used a case study detailing spending 25 hours throughout the process of screening a single patient. Tasks included difficult patients facing interactions such as explaining protocols to patients, justifying the volume of data points being collected on request of sponsors—which are often times unclear in the protocol—and troubleshooting due to faulty equipment or unclear protocols provided by sponsors during patient screening, and breaking the difficult news that they did not meet the inclusion criteria after being hopeful and investing their own time in the process. Additionally, sites aren’t compensated for failed screening—and if they had been compensated, these 25 hours of work would have equated to $75–100. Shockingly, she described this as a normal day for site staff, leaving the question being, are we underestimating the time and effort that sites put into a trial and is the compensation they receive reflective of everything they do?
It is clear that there is discord between sites and sponsors, and more needs to be done to build a partnership that is built on reciprocity and collaboration, to ensure sites are confident and equipped to successfully complete a trial, and the sponsors trust them to do so.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData