Australian mothers and babies are at risk due to a critical shortage of several crucial medicines considered safe for use in pregnancy. Shortages are said to be a result of manufacturing and distribution issues, with high blood pressure medications such as prazosin, clonidine, and nifedipine particularly affected. These blood pressure medications are vital to prevent the effects of uncontrolled, severe gestation blood pressure, which can result in mortality, and even acute high blood pressure, which can lead to strokes and brain haemorrhages. Another vital need for these medications is in the prevention of pre-eclampsia, a dangerous blood pressure-related condition of pregnancy that affects the arteries carrying blood to the placenta, with permanent consequences to both mother and baby.
In Australia, there are only six drugs registered to treat high blood pressure in pregnancy and all are more than 30 years old, so are well past their patent expiry, meaning there is minimal profit to be gained by pharmaceutical companies. In turn, many have opted to discontinue their distribution. This begs the question of whether more needs to be done to incentivise pharmaceutical companies to maintain the supply of older, off-patent drugs, as the lack of distribution and manufacturing by pharmaceutical importers is putting lives at risk. However, no arm of government is responsible for ensuring the supply of such drugs, which is why obstetric experts in the Medical Journal of Australia have proposed a call for action to the federal government to create a publicly funded entity, with the hopes of ensuring a stable supply of treatments for pregnancy conditions including pre-eclampsia, postpartum haemorrhage, and nausea.
This call for action has been backed by the Royal Australian and New Zealand College of Obstetricians and Gynecologists. The systematic exclusion of pregnant women from clinical trials of new drugs is evident when considering there are only six outdated high blood pressure treatments suitable in pregnancy, compared to the more than 50 drugs available for high blood pressure in the non-pregnant population. This stems from valid concerns about the potential impact of medications on pregnant women after the thalidomide tragedy, which saw thalidomide used to treat nausea in pregnant women but resulted in severe birth defects in thousands of children. However, fear of repeating history has pushed too far the other way, where pregnant women are largely excluded from trials.
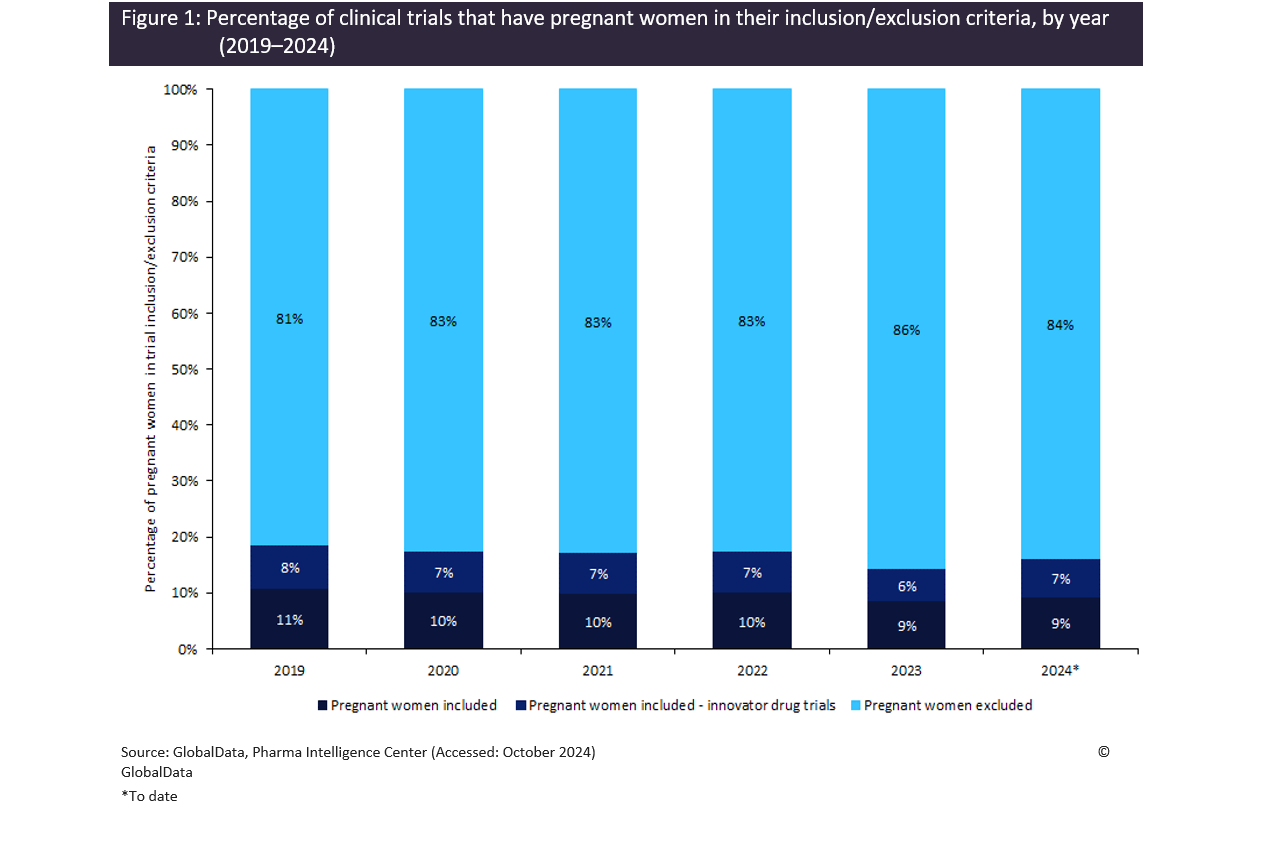
According to GlobalData’s trials intelligence platform, 4% of clinical trials globally initiated in 2024 include pregnant women in their inclusion criteria compared to 36% that have pregnant women in their exclusion criteria, the remaining 60% have no mention of pregnancy in their inclusion or exclusion criteria. Furthermore, the data also indicates the proportion of trials that have pregnant women in their exclusion criteria has steadily increased year-on-year, while the percentage of trials investigating innovator drugs with pregnant women in their inclusion criteria is decreasing (Figure 1). This indicates that this issue is a global issue affecting women beyond Australia, and a global call for action may be warranted to ensure pregnant women are not excluded from potentially life-saving treatments.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData