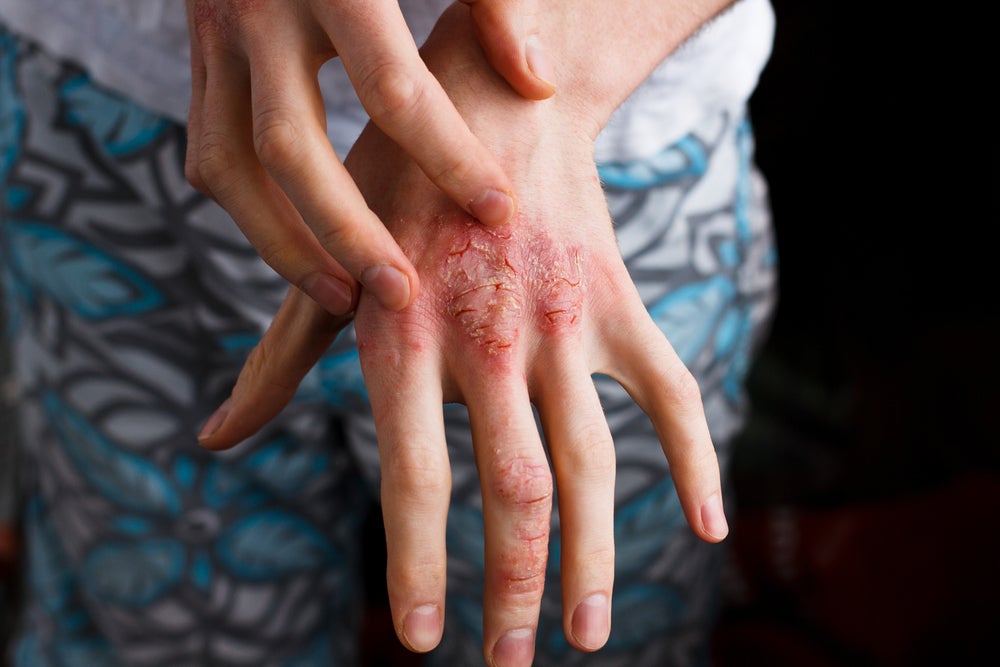
The atopic dermatitis (AD) space has received attention in recent years due to increased disease awareness and severe cases that require effective treatment. AD has a high global prevalence in children and adolescents (approximately 20% of the global population) in comparison to adults (approximately 10%) with different severity levels, highlighting the need for improved treatment management (Langan et al, 2023).
Patients with AD suffer with itchy skin, red rashes, dry and scaly areas, swollen skin, blisters and small rough bumps, all of which affect patients’ quality of life (QoL). AD treatment involves approaches that focus on the elimination of flares, inflammation reduction and repairing the skin barrier, depending on the severity of the disease. Currently, the AD space has numerous unmet needs, many of which are related to the poor efficacy of AD treatments, highlighting the need for new and effective treatments. Recent approvals of multiple topical therapies including Dermavant’s Vtama (tapinarof) and Arcutis’s Zoryve (roflumilast) are considered promising as they employ novel mechanisms of action for the AD space, contributing to improvements to patients’ QoL and potentially addressing unmet needs.
At present, AD treatment management depends on disease severity. Basic treatment focuses on trigger avoidance through bath oils and emollients. In mild cases, as determined by the Eczema Area and Severity Index (EASI) score, emollients are used to restore the skin barrier. Current therapies for AD encompass topical and systemic treatments including inhibitors that target Janus kinases (JAK), phosphodiesterases, calcineurin and interleukins (ILs), as well as the aryl hydrocarbon receptor agonist (AhR) modulators and immunosuppressants. Many of these therapies are used off-label.
During June and July 2024, Dermavant’s Vtama was approved in Japan and Arcutis’s Zoryve was approved in the US. Both treatments possess interesting mechanisms of action (MoAs). Vtama is an AhR agonist and presents a novel mechanism for AD which no other marketed therapies employ. Zoryve is a phosphodiesterase-4 (PDE4) inhibitor, and this MoA has been previously seen in Pfizer’s Eucrisa (crisaborole). Following recent approvals, the AD community is looking forward to promising outcomes from both therapies upon their use in AD patients. The AD space is very dynamic, with multiple pipeline agents currently in clinical trials. The additions of Dermavant’s Vtama and Arcutis’s Zoryve are promising and may provide an additional benefit to AD patients as their unmet needs are further addressed.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData