Clinical trial diversity remained a hot topic throughout Clinical Trial Arena’s global Outsourcing in Clinical Trial (OCT) Conference series at the beginning of 2024.
Critical conversations were at an all-time high during the 11th Annual Outsourcing in Clinical Trials UK & Ireland conference, held on 11-12 June 2024, two weeks before the US Food and Drug Administration (FDA) was set to issue its long-awaited Diversity Action Plan.
During the conference, the lack of transparency around key clinical trials and diversity metrics became a massive talking point.
During a well-attended panel discussion, Andrew Ustianowski, network director for Upcoming Northwest Regional Research Diversity Network from National Institute for Health and Care Research, commented that many people responsible for improving diversity within clinical trials do not even have access to this data on a global scale and emphasised the importance of making it mandatory to disclose this information in clinical trial legislation.
Throughout the panel and several other presentations, the general sentiment was that increasing transparency pertaining to diversity and other trial metrics such as site-specific enrolment data, will be vital for moving the industry forward and holding the system accountable.
Two weeks after the OCT conference, the FDA’s draft guidance ‘Diversity Action Plans to Improve Enrolment of Participants from Underrepresented Populations in Clinical Studies’ was released.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThis was a necessary step towards increasing the enrolment of historically underrepresented populations in clinical studies so that clinical trial populations can be representative of real-world populations.
Following the release of the FDA’s guidance, sponsors must now specify their rationale and goals for clinical study enrolment (separated by the age group, sex, and race of clinically relevant study populations), and describe how they intend to meet those goals for certain studies.
There are also guidelines on how sponsors can submit a request for a waiver from submitting a Diversity Action Plan.
However, in both instances, this new regulation will prompt sponsors to think critically about their intentions regarding the characteristics of the patient population they aim to treat.
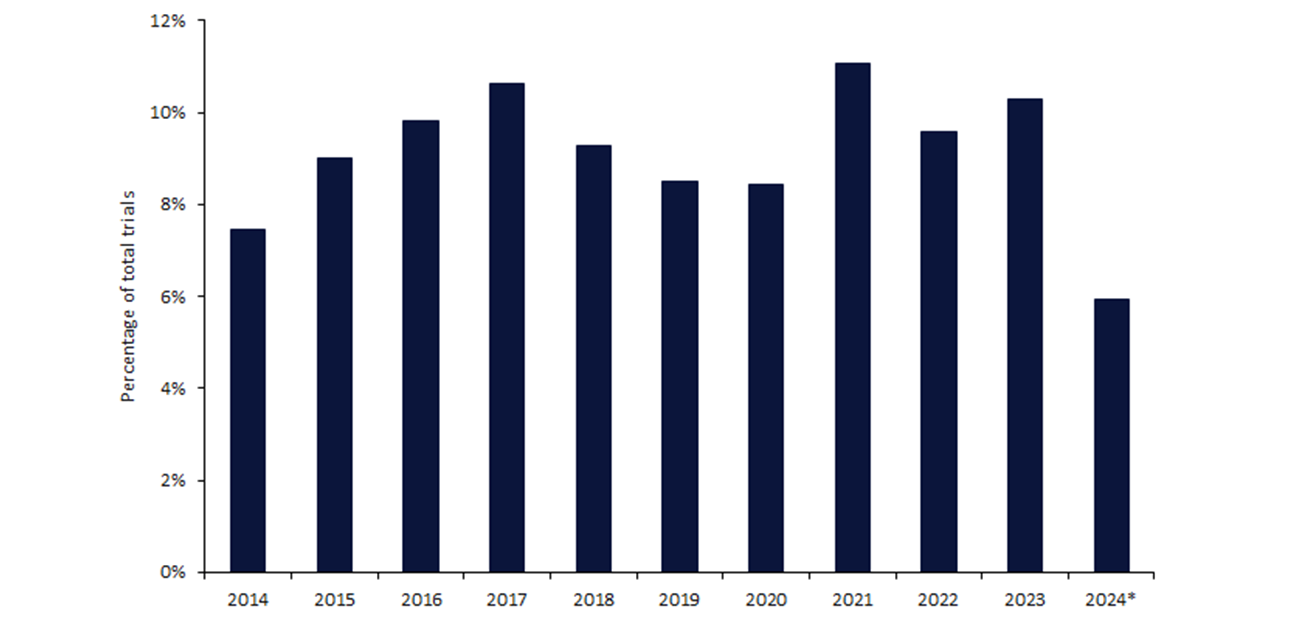
Data obtained from leading data and analytics company GlobalData’s trials intelligence platform indicates that around 8.6% of clinical trials revealed the race of their participants.
The number of studies sharing this information appears to fluctuate year on year around the same figure with minimal increase (Figure 1).
As such, the FDA’s guidance is vital and comes at a critical time.
Hopefully, the quantity of trial-specific diversity data will increase as this guidance takes effect.