At the recent IASLC 2023 World Conference on Lung Cancer (WCLC23), Gilead Sciences announced encouraging results from its Phase II EVOKE-02 study, which evaluated the combination of Trodelvy (sacituzumab govitecan-hziy) and Merck & Co.’s anti-PD-1 therapy Keytruda (pembrolizumab) in patients with previously untreated advanced or metastatic non-small cell lung cancer (NSCLC) without actionable genomic alterations.
The combination therapy aims to enhance anti-tumour activity by leveraging the unique mechanisms of both drugs. Trodelvy is an antibody-drug conjugate (ADC) that targets the TROP-2 antigen. In contrast, Keytruda is a PD-1 inhibitor that modifies the immune response. The synergy could potentially offer a new standard of care in the competitive NSCLC market. Based on PD-L1 tumour proportion score (TPS), the study comprises two cohorts: cohort A with TPS ≥ 50% and cohort B with TPS < 50%. The trial findings demonstrated a noteworthy overall objective response rate (ORR) of 56%, which surpasses the ORR of 44.8% observed in the previous Phase III trial of Keytruda monotherapy (known as KEYNOTE-024). Additionally, the disease control rate (DCR) was 82%. However, it is worth noting that the median duration of response (DoR) was not available at the time of the data analysis.
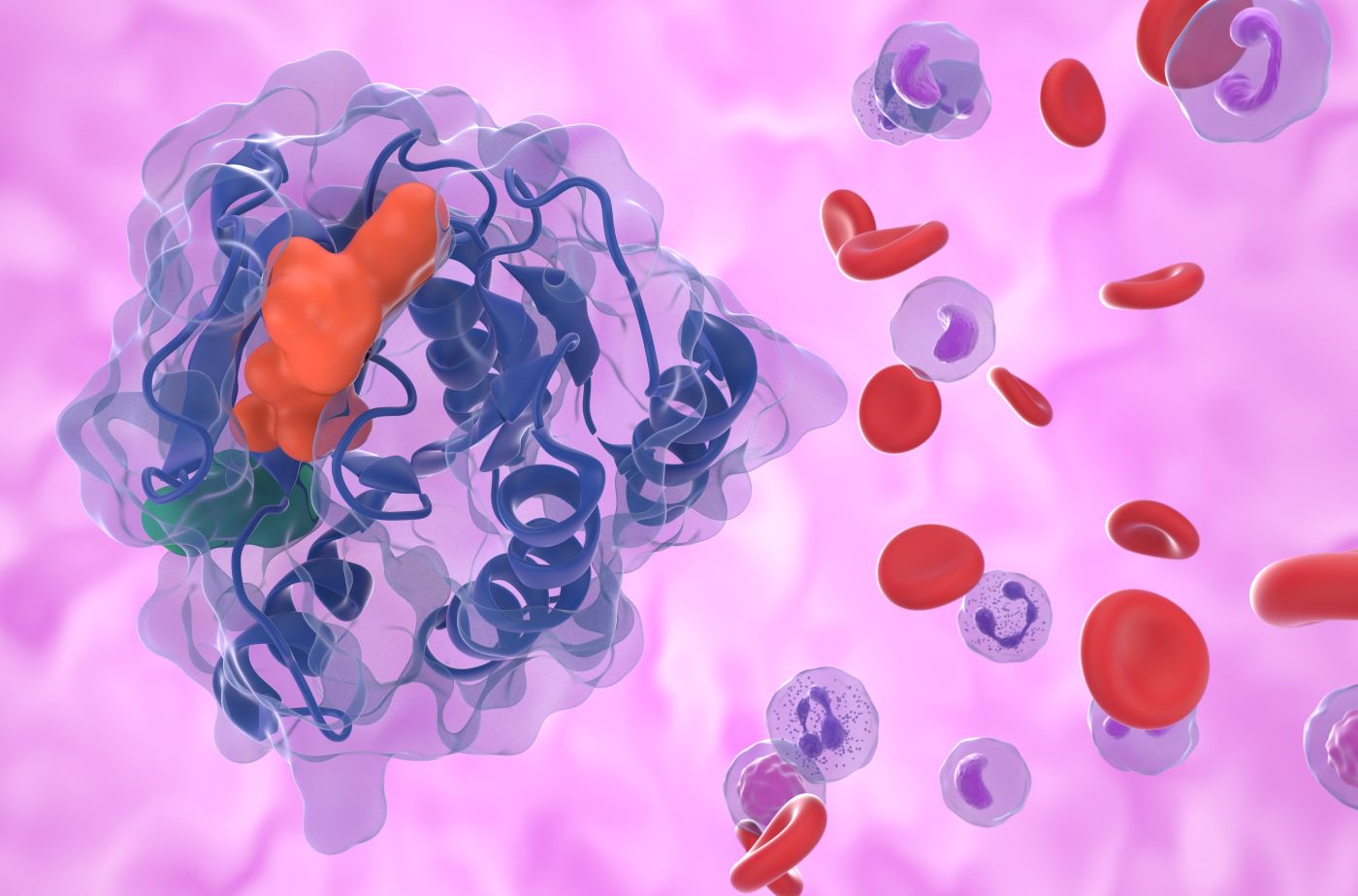
TROP-2 protein is overexpressed in numerous malignancies, including NSCLC. Trodelvy consists of a humanised TROP-2 monoclonal antibody, sacituzumab, linked to a topoisomerase I inhibitor SN-38 via a hydrolysable linker (CL2A). Trodelvy binds to TROP-2 expressing cancer cells. Upon internalization, SN-38 is released, acting to prevent the repair of topoisomerase I-induced single-strand DNA breaks. The DNA damage induces apoptosis and cancer cell death.
According to GlobalData’s consensus Analyst Forecast, global sales for Trodelvy are expected to reach nearly $3bn by 2029, driven by expansion into new indications, including NSCLC, as well as bladder, breast, and ovarian cancers globally. This robust projection is underpinned by Trodelvy’s extensive clinical trials, including 15 Phase III trials as first or subsequent-line therapy for multiple cancers. On the competitive front, Trodelvy will have to contend with AstraZeneca/Daiichi Sankyo‘s datopotamab deruxtecan, which, although not yet approved, has shown encouraging Phase III clinical trial data in NSCLC treatment and has projected sales of $2.5 billion by 2029. Trodelvy leads in clinical scope with 44 ongoing trials, but datopotamab deruxtecan is also advancing with 18 ongoing trials. Furthermore, Trodelvy’s shorter half-life may require more frequent dosing, a potential drawback compared to datopotamab deruxtecan’s longer half-life, which could increase patient compliance. EVOKE-02’s promising results validate Gilead’s approach to the Phase III EVOKE-03 investigation of Trodelvy in combination with Keytruda vs. monotherapy for first-line PD-L1-high metastatic NSCLC patients. Nevertheless, this study is only in Phase II, and it is crucial to be aware of the drug’s associated boxed warning for severe or life-threatening neutropenia and severe diarrhoea. In addition, Trodelvy contains a genotoxic component, SN-38, with embryo-fetal toxicity. These life-threatening adverse effects may limit Trodelvy’s market penetration, as clinicians must be cautious when prescribing to a diverse patient population.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData