The US approval of Eli Lilly’s Ebglyss (lebrikizumab) in September 2024 places the company in a competitive position in the atopic dermatitis (AD) market.
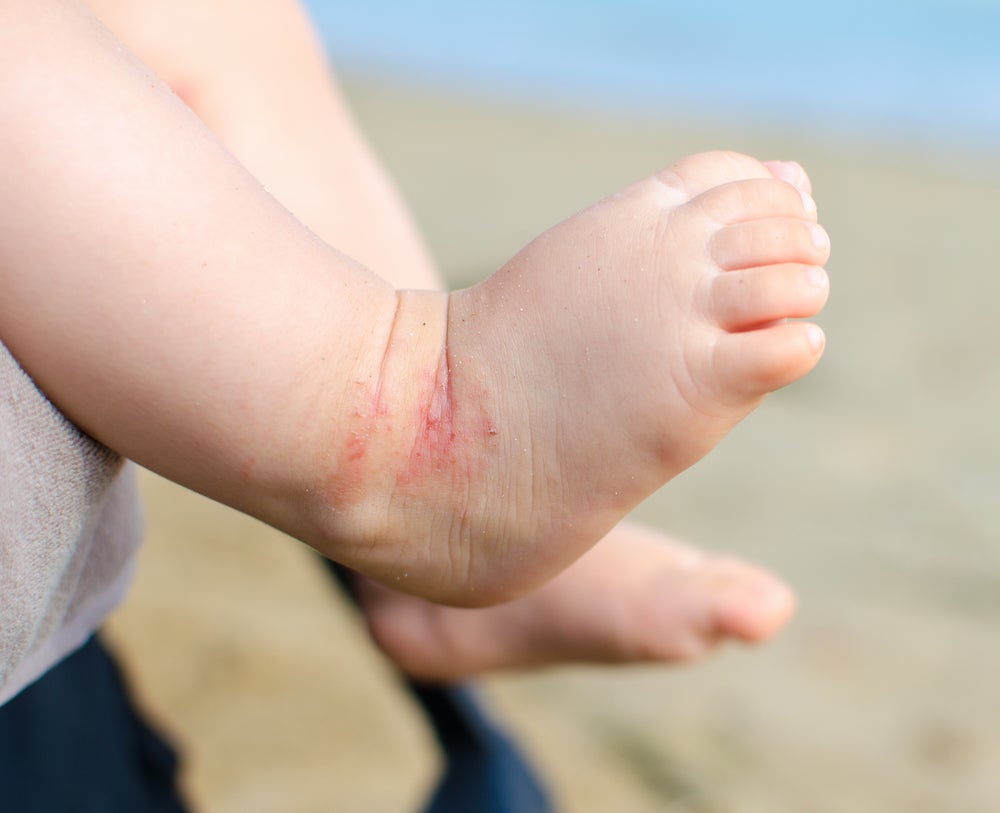
AD, also known as eczema, is a chronic inflammatory skin condition characterised by itchy, red and swollen skin. The condition can significantly impact quality of life due to its symptoms and the need for ongoing management. Symptoms typically include dry skin, itching, redness and the appearance of rashes. Current treatment options for AD range from topical therapies such as corticosteroids and emollients to systemic treatments, including immunosuppressants and biologic drugs. Despite currently available treatments, a significant unmet need remains for safer and more effective long-term AD therapies, which may be further addressed by this new approval in the US.
The entry of Ebglyss in the AD market marks a significant milestone, as it offers a therapeutic option for AD patients who have had limited success with existing treatments in the US. Ebglyss’s pivotal clinical trials, ADvocate 1 (NCT04146363) and ADvocate 2 (NCT04178967), demonstrated its therapeutic efficacy in reducing the signs and symptoms of AD. It was found to be generally well-tolerated and most adverse events were mild-to-moderate in severity, including injection site reactions, conjunctivitis and risk of skin infections. The incidence of trial discontinuation due to adverse events was low. Ebglyss is anticipated to directly compete with other therapies with similar mechanisms of action (MoAs) such as Sanofi/Regeneron’s Dupixent (dupilumab) and Leo Pharma’s Adbry/Adtralza (tralokinumab).
Ebglyss set to capture market share
Ebglyss, as with Dupixent and Adbry/Adtralza, works by disrupting the forming of the interleukin (IL) and receptor complex, and thereby blocking the subsequent cellular pathways. However, while Dupixent targets both the IL-4 and IL-13 pathways, Ebglyss and Adbry/Adtralza selectively target IL-13. Comparative studies have investigated the different biologics, with in vitro studies suggesting that lebrikizumab exhibits a higher binding affinity and a slower rate of dissociation than tralokinumab (Okragly et al., 2021). Furthermore, a study indirectly comparing lebrikizumab and dupilumab in patients with moderate-to-severe AD suggested that lebrikizumab may provide equal or superior long-term efficacy, as measured by the Eczema Area and Severity Index (EASI-75) and the Investigator Global Assessment (IGA) 0/1, with the advantage of requiring less frequent doses (Rand et al, 2024).
Another study involving an indirect comparison of tralokinumab and dupilumab demonstrated a similar efficacy across clinical response endpoints at week 32 (IGA 0/1, tralokinumab 49.9% vs dupilumab 39.3%; EASI-50, 78.9% vs 77.5%; EASI-75, 71.5% vs 71.9%; EASI-90, 53.3% vs 56.2%) (Torres et al, 2024). Nevertheless, more comparative studies between Ebglyss, Dupixent and Adbry/Adtralza will be crucial in determining the relative efficacy and safety profiles. Such data will further guide clinicians in personalised treatment decisions and could influence patient preference.
Success will depend on product differentiation
With a similar MoA, as well as its demonstrated efficacy and safety, Ebglyss is expected to capture market share from Dupixent and Adbry/Adtralza, contributing to the overall growth of the AD treatment market. Ebglyss may also possess the advantage of less frequent dosing, as its maintenance dose is a subcutaneous 250mg injection every 4 weeks, compared to Dupixent and Adbry/Adtralza which have a dosing regimen of 300mg administered subcutaneously every 2 weeks. Nevertheless, as Ebglyss enters a competitive landscape with established and emerging therapies vying for market share, its success will depend on its differentiation from other treatments and strategic positioning by its manufacturer.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe separation of Ebglyss from Dupixent and Adbry/Adtralza will rely on its real-world clinical benefits, patient experience and cost-effectiveness. Strategic market positioning will be essential to capitalise on these points of distinction. However, Ebglyss’s uptake is expected to be both slow and subject to clinician and patient preference, as with all newly approved therapies. Reimbursement by insurers and accessibility for patients will also influence its competitive edge globally, particularly in markets that are sensitive to healthcare costs.
Ebglyss’s approval highlights the opportunity that exists in the AD space, and may have potential advantages in efficacy and safety against AD, expanding the therapeutic options currently available for those patients. Monitoring emerging trends and patient outcomes will be essential to understand the evolving landscape of AD treatments, especially when biologic treatments provide a costly but efficacious way to tackle the disease.