The rapid surge of mpox (formerly known as monkeypox) has prompted the World Health Organization (WHO) to sound its highest level of alert and declare a public health emergency of international concern (PHEIC), with many scientists drawing resemblance to the early days of HIV due to severity, unknowns, and stigma. This alert means that access to testing, vaccines, and therapeutic drugs is accelerated in affected areas. The WHO has released $1.5m so far from its contingency fund to support containment efforts in the most affected regions, including the Democratic Republic of Congo (DRC) and neighbouring countries, that have been hit by the emergence of a new type of the virus coined clade IB—an offshoot of clade I, whose outbreak started in the DRC in January 2023 and has since reached 12 other countries in the region. This is even more worrying than the 2022 outbreak of mpox that led to around 100,000 cases worldwide as clade II—the less transmissible and severe version—was at the root of the spread. Historically, clade I has been deadlier than clade II, which is contributing to the WHO’s urgent approach to the outbreak.
Currently, there is no treatment approved specifically for monkeypox virus (MPXV) infections. For many people, mpox is treated with supportive care for symptoms such as pain and fever, and in those severely affected, antiviral drugs such as cidofovir or tecovirimat, approved for the treatment of other viral infections such as smallpox, are utilised and deemed safe—although the extent of their efficacy and durability in mpox is yet to be determined. A vaccine for mpox is desperately needed as immunisation will likely be a crucial technique for controlling mpox in humans. China has recognized this and is at the forefront of innovation for mpox, as the Shanghai Institute of Biological Products’s independently developed mpox vaccine candidate, based on a live, attenuated strain called MVA, was cleared on 9 September to enter clinical trials. Hopefully, this will have the potential to be the country’s first experimental dose to battle this deadly disease.

Access deeper industry intelligence
Experience unmatched clarity with a single platform that combines unique data, AI, and human expertise.
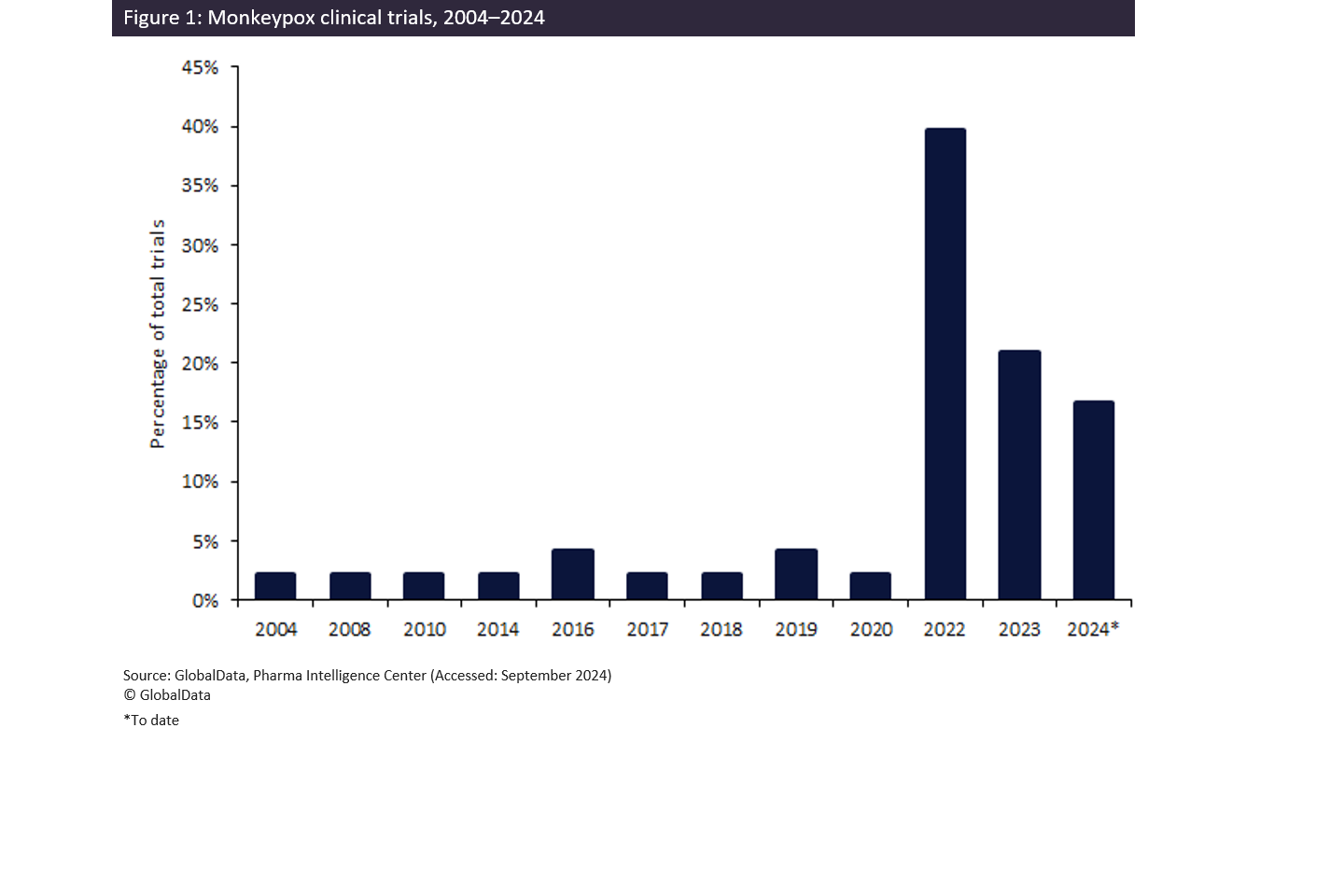
According to GlobalData’s trials intelligence platform, there are 42% more smallpox trials than mpox trials—although both figures remain low. According to Figure 1, there was a spike in monkeypox trials in 2022 due to the previous outbreak, but figures then declined in 2023. It is likely that there will be another spike in 2024, as the number of trials is almost exceeding 2023 figures, with the full response from pharmaceutical companies still to be reflected in 2024 figures considering the latest outbreak. Although figures have increased by 88% from 2004 to today, global research efforts will need to step up to bring us to a more stable position with this deadly disease.