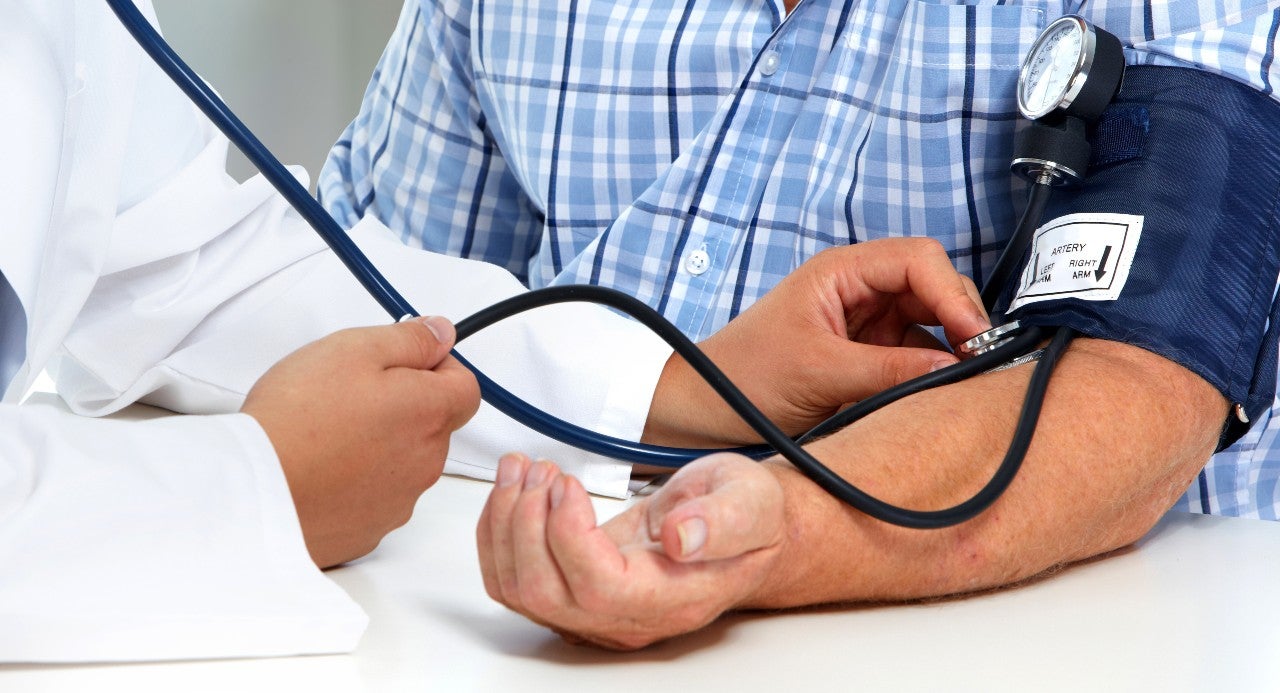
ReCor Medical’s Paradise ultrasound renal denervation (RDN) system has recently received Breakthrough Device Designation from the FDA. If approved, the Paradise system could be a novel approach for the management of hypertension patients, particularly among those with severe disease who are resistant to standard-of-care drug therapies. Therefore, it has the potential to address a strong unmet need in the market.
The Paradise system is a catheter-based system that is being investigated in patients with uncontrolled hypertension who are inadequately responsive to anti-hypertensive medications. The device targets overactivity in the renal nerves, which have an established role in the pathogenesis of hypertension. The system is inserted into the renal artery via a small catheter. Ultrasound energy is delivered to the tissue surrounding the artery, which generates heat. This causes the denervation of the overactive renal nerves, which, in turn, lowers blood pressure.

Access deeper industry intelligence
Experience unmatched clarity with a single platform that combines unique data, AI, and human expertise.
Data generated from the RADIANCE-HTN TRIO trial—a randomised, double-blind, sham controlled, two-cohort study—are encouraging. The trial enrolled 136 patients who had uncontrolled hypertension despite ongoing treatment with a single-pill combination drug comprised of a calcium-channel blocker, an angiotensin II-receptor blocker, and a diuretic. The trial met its primary efficacy endpoint, with patients in the Paradise system arm achieving a greater reduction in daytime blood pressure between baseline and two-month follow-up, relative to patients in the sham procedure arm. Furthermore, in the earlier RADIANCE-HTN SOLO trial, the Paradise system was found to effectively lower blood pressure in patients with untreated hypertension.
RDN techniques have been investigated for the treatment of hypertension for several years. While early studies appeared promising, the field suffered a significant setback in 2014 due to the first randomised, blinded, sham-controlled trial, named SYMPLICITY HTN, failing to meet its primary efficacy endpoint. RDN largely fell out of favor following the publication of these results. However, the RDN field has witnessed a resurgence in recent years. In addition to ReCor Medical, several other companies are investing in RDN devices including Medtronic, which has published positive results for its RDN device in resistant hypertension, as well as untreated hypertension.
The renewed interest in RDN is exciting considering the enormous disease burden associated with this condition. GlobalData estimates that the diagnosed prevalence of hypertension in 2020 is 156,299,033 cases across the seven major markets (7MM) (US, France, Germany, Italy, Spain, UK, and Japan). Although the antihypertensive drugs market is crowded, there are still a considerable number of unmet needs in this space. Treatment compliance is often poor due to the side effects associated with these drugs. Furthermore, even in patients who are highly compliant, hypertension may remain inadequately controlled. For example, in a US study of over 4,000 patients with hypertension, 19.7% were found to be inadequately controlled despite treatment with multiple antihypertensive drugs.
This highlights the need for innovative non-pharmacological approaches such as RDN for the treatment of resistant hypertension. Having won Breakthrough Device Designation for the Paradise system, ReCor Medical is now in a better position to capitalize on this market gap.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData