The recent groundbreaking approval of Takeda’s Eohilia for patients 11 and older by the US Food and Drug Administration (FDA) may change the current treatment landscape for eosinophilic oesophagitis (EoE), with Eohilia now being the only competitor of Sanofi’s Dupixent, which was once the only approved therapy against EoE in the US.
Before Takeda’s Eohilia approval, the Japanese manufacturer had undergone a lengthy regulatory process with hopes of eventually receiving approval from the FDA.
The approval comes months after Takeda’s New Drug Application resubmission for Eohilia to the FDA, following feedback received by the latter.
Takeda’s Eohilia approval marks a significant achievement for the pharmaceutical company in the field of EoE, which has the potential to capture market share from Dupixent for the treatment of EoE.
Nevertheless, unlike Eohilia, Sanofi’s Dupixent is the only approved therapy for EoE in patients 11 and below, and therefore Eohilia will have to prove efficacy in this age group in future studies as well.
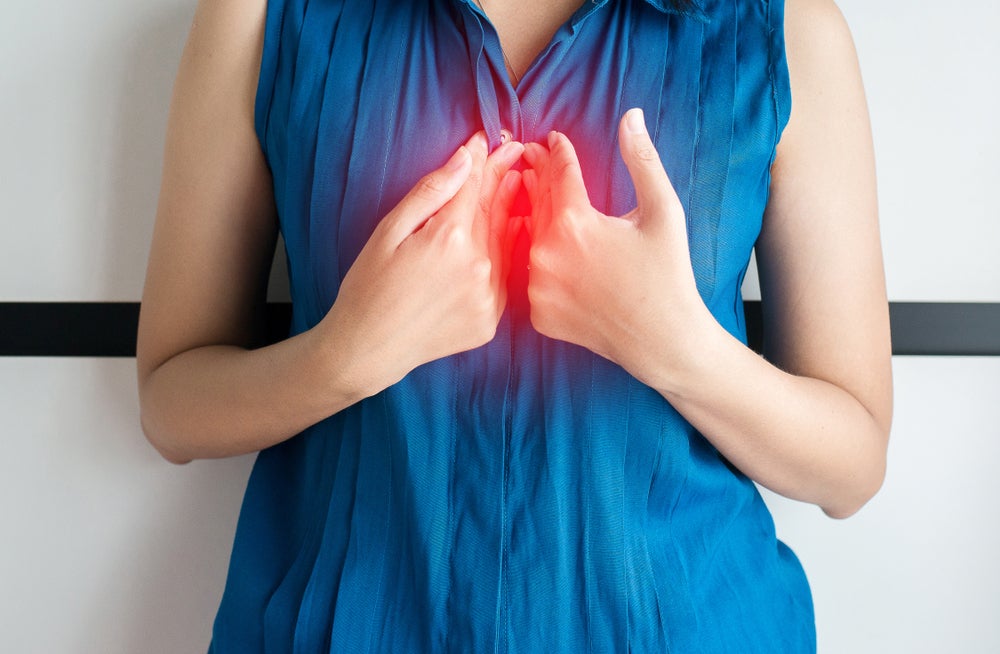
EoE is a chronic disease, impacting the normal function of the oesophagus through immune-mediated reactions that stimulate inflammatory responses.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataEoE patients exhibit symptoms including feeding refusal, vomiting, and dysphagia, which heavily impact the quality of life for patients.
Eohilia is an oral corticosteroid that has been shown to have efficacious effects in patients with EoE, according to preliminary results of studies demonstrating the superiority of Eohilia in achieving improved histologic remission, compared to placebo.
Prior to Takeda’s Eohilia approval, the only available treatment in the US was Sanofi’s Dupixent, which is a subcutaneous monoclonal antibody therapy against EoE.
After the recent news of Eohilia’s approval, Sanofi was looking to reinforce Dupixent’s standing in the market by showing promising data on the effect of Dupixent in patients with EoE at the 2024 American Academy of Allergy, Asthma & Immunology annual meeting held on 23-26 February 2024 demonstrating its potential in transforming the treatment landscape for EoE patients, with a focus on the pediatric group, according to a 9 February press release.
As of today, Eohilia is the only oral therapy approved for patients 12 and above in the US, maintaining a competitive advantage over Sanofi’s Dupixent subcutaneous therapy, with studies highlighting the increased preference of patients choosing oral over subcutaneous therapies.
Nevertheless, Dupixent’s approval has also considered the paediatric population (1-11 years old), compared with Eohilia, which can be considered an advantage within the landscape of EoE treatment.
Looking forward, the EoE landscape may be further saturated by other pipeline agents that are currently in late-stage clinical trials.
According to leading data and analytics company GlobalData’s Pharma Intelligence Center, Bristol Myers Squibb’s intravenous cendakimab, as well as Ellodi Pharmaceuticals’ oral fluticasone propionate, are in Phase III clinical trials for EoE in the US, and upon a potential approval, these companies also stand a chance to acquire further market share from Takeda and Sanofi in the EoE field.
These potential entries may further impact the size of the EoE treatment market positively, highlighting opportunities in the field.