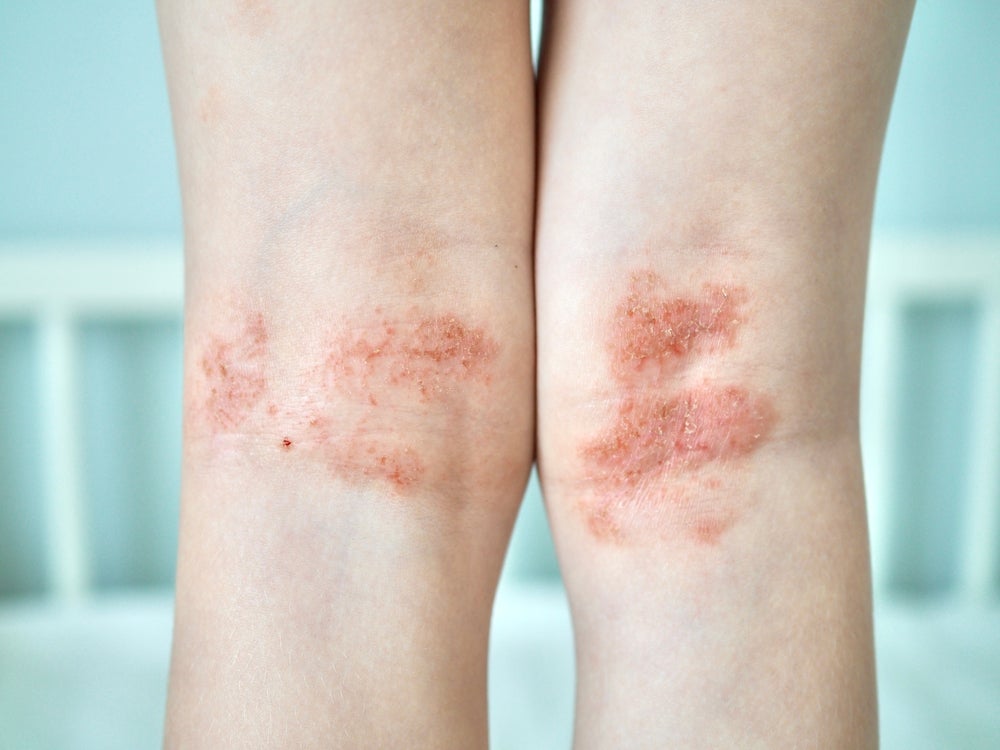
Following the recent positive results of the Phase III clinical trial for Dermavant’s VTAMA (tapinarof) on atopic dermatitis (AD) patients of two years of age and older, the pharmaceutical company based in the US has received FDA acceptance of the drug’s Supplemental New Drug Application (sNDA). This marks a milestone for Dermavant, as it is one step closer to market authorization for their asset for the treatment of AD patients two years of age and older. Tapinarof is an aryl hydrocarbon receptor (AhR) receptor modulator that has been observed to have anti-inflammatory effects and has been marketed for plaque psoriasis, with clinical studies also showcasing tapinarof as a promising approach for the treatment of AD. Tapinarof is a first-in-class molecule that employs the binding of AhR to decrease proinflammatory cytokines, decrease oxidative stress, and promote skin barrier normalization. According to GlobalData’s Pharmaceutical Intelligence Centre (PIC), the pipeline landscape for AD currently includes biologics, oral small molecules, anti-puritics, and topical small molecules. Key mechanisms of action (MoAs) seen among these topical small molecules include Janus kinase (JAK) inhibitors and phosphodiesterase-4 (PDE4) inhibitors. Opportunity is found within the AD field, specifically in drugs that can target the AhR for the treatment of AD, as observed from tapinarof’s clinical trials, highlighting the potential patient share that can be captured, considering the low adverse effect occurrence seen in previous clinical trials.
Tapinarof is a nonsteroidal, topical AhR-modulating agent that can result in the modulation of type 17 helper T cytokines through activating the nuclear factor erythroid 2‐related factor 2 (NRF‐2) pathway, consequently producing antioxidative enzymes that suppress oxidative stress and thus inflammatory pathways. Dermavant’s VTAMA has been marketed for adult patients with plaque psoriasis and was first approved in May 2022 by the FDA. Tapinarof’s MoA seems promising, with clinical trials showing good tolerance with minimal adverse effects in patients with AD, hinting at an opportunity for future therapies that may employ a similar mechanism.
GlobalData’s PIC has identified a pipeline landscape for topical treatments against AD; innovator assets that are currently in Phase III and pre-registration include Jiangsu Hengrui Medicine’s ivarmacitinib sulfate (China) and Arcutis’s roflumilast (US, Canada), respectively. Other innovator topical pipeline assets that are currently in Phase III development include Suzhou Zelgen Biopharmaceutical’s jaktinib hydrochloride, Minghui Pharmaceutical’s MH-004, Maruho’s M-6100, Beijing Puqi Pharmaceutical Technology’s PG-011, Shanghai Thederma Pharmaceuticals’s TAP-1503, and Tianjin Institute of Pharmaceutical Research’s diflucortolone. In addition, GlobalData has estimated tapinarof’s sales to reach $766m by 2030 globally for AD, subject to approval. This, in combination with the high competition in therapy development, highlights the opportunity for companies to acquire patient share in the AD field.