Ultragenyx has terminated its Phase I/II trial for pipeline candidate UX053, a non-vaccine mRNA therapeutic for glycogen storage disease type III, also known as Cori’s disease. The study was terminated due to reasons unrelated to safety concerns, although the specific rationale has not been disclosed by the biopharmaceutical company. UX053 was one of five non-vaccine mRNA therapeutics currently in clinical trial development; the other four are all in Phase I/II. mRNA therapeutics hold a lot of promise because of the technology’s potential applicability to a wide range of disorders. However, proving the efficacy of these drugs could be difficult, especially for rare diseases in which participant recruitment is tricky.
UX053 is an antisense oligonucleotide to be intravenously administered. Participants received a single peripheral intravenous infusion of UX053. After completion of a 90-day follow-up period, study participants could then elect to enter the open-label repeat-dose cohort to receive a dose of UX053 every four weeks for four doses. Cori’s disease is an inherited rare disorder caused by the buildup of glycogen in the body’s cells, impairing organ and tissue function throughout the body but especially in the liver and muscles. Other rare indications that are being explored in non-vaccine mRNA clinical trial development include methylmalonic acidemia and propionic acidemia.
The mRNA therapeutic space has erupted since mRNA technology proved instrumental in fighting the Covid-19 pandemic. Now, pharmaceutical companies are exploring mRNA technology’s potential in other disease areas. mRNA therapeutics involve the delivery of in vitro transcribed (IVT) mRNA into a target cell, where cellular machinery translates the mRNA into a functional protein. This allows for the transient expression of proteins that are absent, dysfunctional, or have been suppressed in various diseases. mRNA therapies are novel because they can be used for precise and individualized therapy based on a patient’s specific DNA. There are three main therapeutic modalities that utilise mRNA: replacement therapy, in which mRNA is administered to compensate for a defective gene or protein; vaccination, where mRNA is administered to elicit protective immunity; and cell therapy, whereby mRNA is transfected into the cells ex vivo to alter cell phenotype or function. These cells are then delivered to the patient. Moderna, BioNTech, and Omega Therapeutics are all developing non-vaccine mRNA therapeutics for rare diseases and oncological disorders.
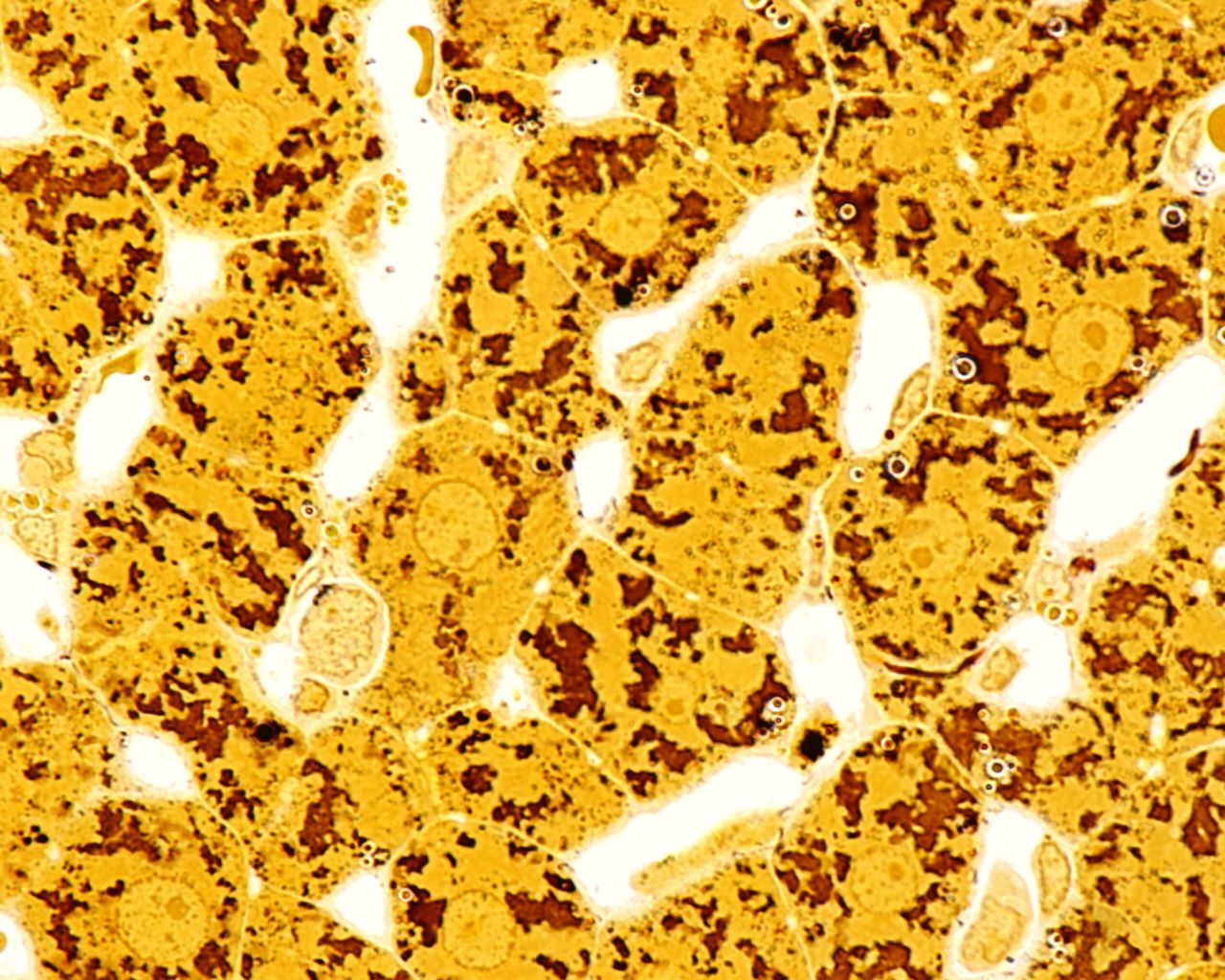
BNT-141 is BioNTech’s pipeline candidate for ovarian cancer, bile duct cancer, adenocarcinoma of the gastroesophageal junction, colorectal cancer, pancreatic cancer, gastric cancer, and solid tumours. The drug is a claudin 18.2 inhibitor, a stomach-specific isoform of claudin-18 that is highly expressed on many tumour cells and is involved in tumour development and progression. By inhibiting claudin-18.2, BioNTech’s pipeline drug can elicit anti-tumour activity. The drug is to be intravenously administered and the trial has an estimated completion date of September 2024. Moderna is developing two therapeutics, mRNA-3705 and mRNA-3927. mRNA-3705 is targeting methylmalonic acidemia as a methylmalonyl-CoA mutase mitochondrial activator, while mRNA-3927 is being developed for propionic acidemia. This drug alleviates the condition by activating propionyl-coenzyme A carboxylase. Finally, Omega Therapeutics’ OTX-2002 is in clinical trial development for hepatocellular carcinoma and solid tumours. The candidate is a Myc oncogene protein inhibitor. When asked by GlobalData about the non-vaccine mRNA therapeutics in development, key opinion leaders indicated they believe OTX-2002 has the most potential.
“[OTX-2002] would be my best bet of what would probably work the best.…[Hepatocellular carcinoma] is a liver cancer. These platforms tend to go to the liver. The hepatocytes, when they are healthy, tend to produce protein and enzymes really well. That’s what the liver is designed for. So, if I were to guess which [pipeline therapy] would be most exciting, [this one] has the most obvious potential for success.”
US Key Opinion Leader

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataHopefully, BioNTech, Omega Therapeutics, and Moderna’s non-vaccine mRNA therapeutics do not follow Ultragenyx’s UX053 with early termination.