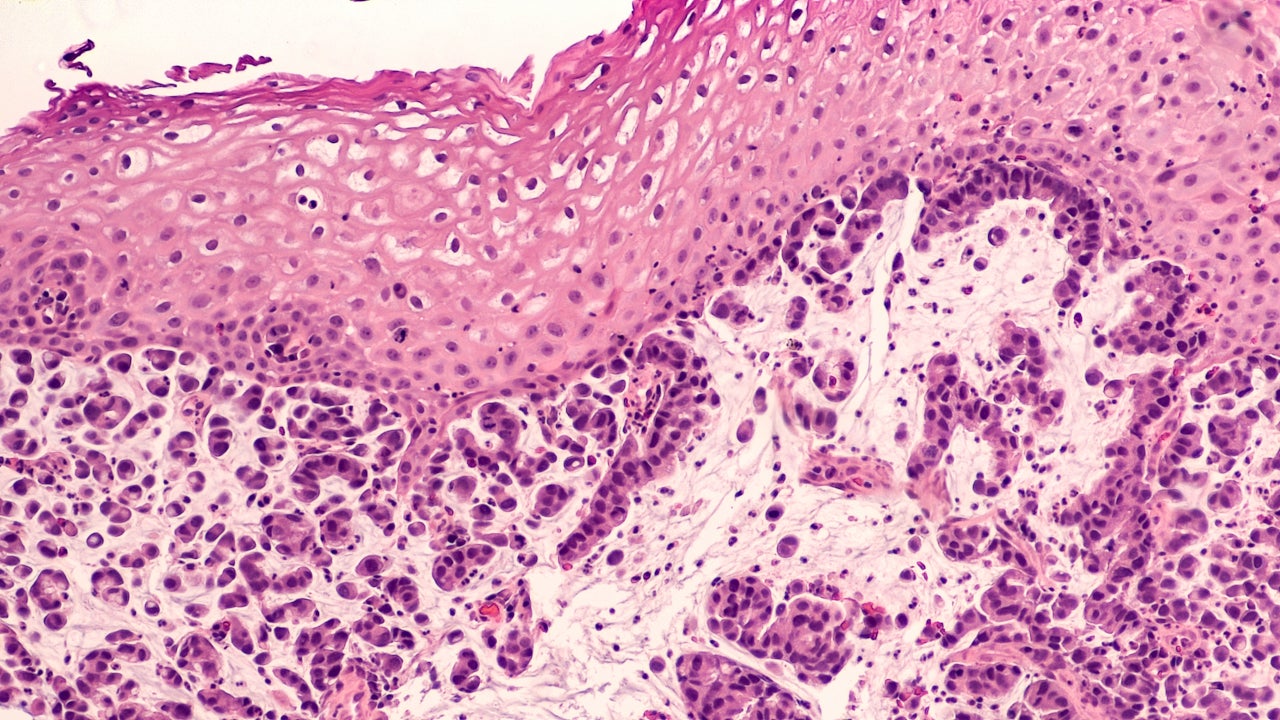
As of 29 January, the US likelihood of approval (LoA) for BeiGene’s Phase III anti-PD-1 antibody for esophageal squamous cell carcinoma (ESCC) rose 10 points, according to GlobalData’s LoA data. The score change was based on topline results of the RATIONALE 302 trial of tislelizumab compared to chemotherapy in patients with advanced unresectable or metastatic ESCC who received prior systemic treatment.
On 27 January, the company announced that RATIONALE 302 demonstrated a statistically significant improvement in overall survival in the intention-to-treat (ITT) population for tislelizumab versus chemotherapy. The therapy’s safety profile was reportedly consistent with its known risks, with no new safety signals noted. BeiGene said it plans to discuss the RATIONALE 302 data with global health authorities and present data at a forthcoming medical conference.
While the LoA prior to this news was 61%, GlobalData’s analysis using a combination of machine learning and a proprietary algorithm has upped the LoA to 71%. The trial is the fourth positive Phase III for tislelizumab and the first in the Phase III programme for gastrointestinal cancers composed of liver, stomach and ESCC, according to the company statement. Global sales are predicted to be $1.4bn by 2026, as per GlobalData consensus forecasts. The company’s market cap is H$260.43bn ($33bn).
by Jennifer C. Smith-Parker is Senior Editor for Pharmaceutical Technology parent company GlobalData’s investigative journalism team. A version of this article originally appeared on the Insights module of GlobalData’s Pharmaceutical Intelligence Center. To access more articles like this, visit GlobalData.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData