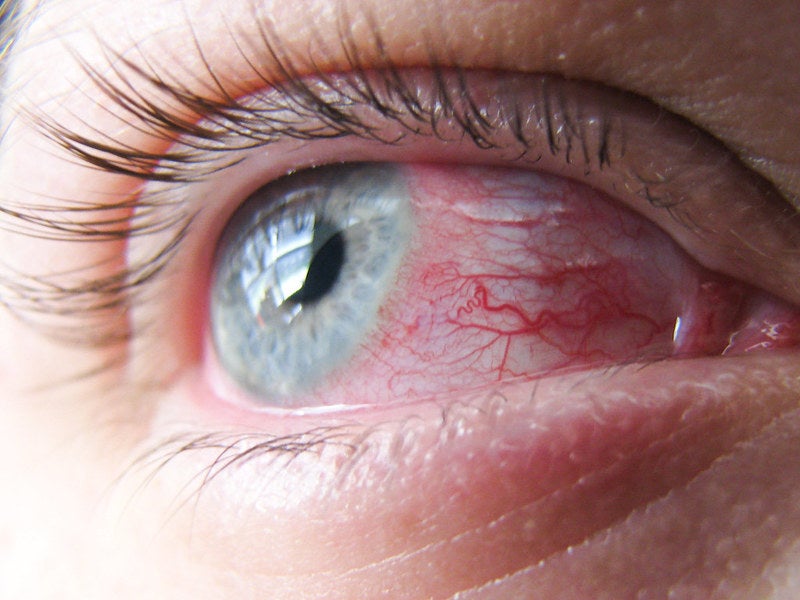
Aldeyra’s ALLEVIATE trial of reproxalap eyedrops meets endpoints
Aldeyra Therapeutics reported positive data from the Phase III ALLEVIATE trial that evaluated the efficacy and safety of 0.25% and 0.5% concentrations of reproxalap topical ophthalmic solution to treat patients with allergic conjunctivitis.
The double-masked, randomised, vehicle-controlled, multi-centre, parallel-group conjunctival allergen challenge ALLEVIATE trial met the primary endpoint and the key secondary endpoint for both reproxalap concentrations.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The primary efficacy endpoint of the trial was the evaluation of ocular itch score area under the curve from ten to 60 minutes after allergen challenge, and the key secondary endpoint was two-point responder rate.
NIH launches Phase I trial of new drug to treat opioid cravings
The US National Institutes of Health (NIH) initiated a Phase I clinical trial at its Clinical Center to assess a new drug candidate, ANS-6637, for treating cravings associated with opioid use disorder (OUD).
The trial is being conducted in alliance with NIH National Institute of Allergy and Infectious Diseases (NIAID) and funded through the agency’s Helping to End Addiction Long-Term (HEAL) initiative.
Opioid use is linked to potential irresistible cravings for opioids, as well as related cues such as injecting equipment or drug use partners.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataBiogen and Eisai end two Alzheimer’s drug trials
Biogen and Eisai decided to scrap the Phase III ENGAGE and EMERGE clinical trials evaluating aducanumab for the treatment of mild cognitive impairment due to Alzheimer’s disease, and mild Alzheimer’s dementia.
The move comes after a futility analysis by an independent data monitoring committee revealed that the trials were not likely to meet their primary endpoint upon completion.
Biogen and Eisai noted that the decision is not based on safety concerns with the drug.
Amgen, Cytokinetics and Servier heart failure drug trial to continue
Amgen, Cytokinetics and Servier announced that their Phase III GALACTIC-HF clinical trial of heart failure drug, omecamtiv mecarbil, passed an interim futility analysis.
Futility evaluation is conducted to check if the trial would demonstrate a positive outcome, and could result in trials being halted early.
A Data Monitoring Committee (DMC) reviewed GALACTIC-HF data and recommended that the trial could continue without any changes.
FINCH 3 trial of filgotinib to treat RA meets primary endpoint
Gilead Sciences and Galapagos announced positive results from the Phase III trial (FINCH 3) of filgotinib to treat adults with moderately-to-severely active rheumatoid arthritis (RA).
The trial evaluated the investigational, oral, selective JAK1 inhibitor filgotinib in combination with methotrexate and as monotherapy in MTX-naïve patients.
FINCH 3 is an ongoing, randomised, double-blind, active-controlled study and achieved its primary endpoint in the proportion of patients achieving an American College of Rheumatology 20% response (ACR20) at week 24.
Gritstone begins treatment in GRANITE-001’s Phase I/II trial
Gritstone Oncology commenced dosing of patients in a Phase I/II clinical trial evaluating its personalised immunotherapy candidate GRANITE-001 in advanced solid tumours.
GRANITE-001 is designed to target and trigger significant T-cell response against the patient’s tumour-specific neoantigens, which will be detected using Gritstone’s EDGE artificial intelligence platform.
The therapeutic comprises a priming adenoviral vector and a monthly boosting with an RNA vector. Both vectors contain the same 20 patient-specific tumour-specific neoantigens.
Breath Therapeutics initiates Phase III BOSTON trials of L-CsA-i
Breath Therapeutics initiated a Phase III BOSTON clinical trials to evaluate the efficacy and safety of its drug candidate Liposomal Cyclosporine A for Inhalation (L-CsA-i) for the treatment of Bronchiolitis Obliterans Syndrome (BOS).
BOS is caused by T-cell mediated inflammation that leads to blockage of bronchioles, the small and medium airways in the lungs, thereby resulting in respiratory failure.
L-CsA-i is a liposomal formulation of cyclosporine A for inhalation administered through a drug-specific investigational eFlow nebuliser and has received orphan drug designation from the Food and Drug Administration and European Medicines Agency to treat BOS.
Phase II trial of SFX-01 meets primary endpoints to treat cancer
Evgen Pharma announced positive headline results from the open-label Phase II STEM trial of SFX-01 to treat patients with estrogen-positive (ER+) metastatic breast cancer.
The STEM trial, which enrolled a total of 46 subjects, evaluated the safety and tolerability of SFX-01 in combination with an aromatase inhibitor (AI) or tamoxifen or fulvestrant.
Evgen is planning further development of SFX-01 for earlier stage patients as an adjunct to a second line hormone therapy to delay the onset of resistance.
Nuformix starts clinical trial of NXP001 to treat CINV
Nuformix started a clinical trial of its lead asset NXP001, which is currently being developed as a treatment for chemotherapy-induced nausea and vomiting (CINV).
First dosing of the cocrystal-based product NXP001 took place recently in a cross-over study that aims to measure the relative bioavailability of NXP001 compared to Merck’s Emend in healthy subjects.
Nuformix plans to complete dosing of patients next year and expects to obtain results by the end of the first half of this year.
Innovent doses first patient in Phase IIa trial of IBI306 to treat hypercholesterolemia
Innovent Biologics announced that the first patient has been successfully dosed in a Phase IIa clinical study of IBI306, a recombinant anti-proprotein convertase substilisin/kexin type 9 (anti-PCSK9) monoclonal antibody, to treat hypercholesterolemia.
The placebo-controlled, double-blind, randomised, multiple ascending dose clinical trial is being conducted in China.
Under repeatedly different doses, the trial will evaluate the efficacy, tolerability, safety, as well as pharmacodynamics (PD) and pharmacokinetics (PK) profiles of IBI306.



