
Among all the current technological advancements which are accelerating the industry, the patient must stay at the heart of every decision that is made was the main theme that flowed through the Summit for Clinical Ops Executives (SCOPE) 2024 conference.
The SCOPE Summit 2024 was held over four days in Orlando, Florida, US, from 11 February until 14 February, and is the premier event for clinical trial strategy and operations.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Throughout the event, there were more than 20 different tracks hosting talks, panels, and presentations, each looking beyond the question of ‘if’ and more about whether things should be done, taking the patient at the heart of those decisions.
From artificial intelligence (AI) and machine learning (ML) to device monitoring, sustainability, outsourcing and decentralising trials, discussions touched on some of the most pivotal decisions which sponsors, biotechs, CROs and sites have to make when conducting research.
Putting patients first is the main objective
The conference kicked off with a session focused on integrating research into the care continuum. The panel discussed how to bring more patients into clinical research, with Christoph Koenen, head of clinical development and operations at Bayer, saying that the industry needs to shift its approach to recruitment.
“I think the basic problem has been in the past that we’ve always expected the patient to come to trial, the patient to come to the investigator and we’ve put a lot of effort and energy into making this happen,” says Koenen.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData“If we bring the trial to the patient, if we integrate the trial into the continuum of care as much as possible, at some point, we will find ourselves in a situation where patients do not even notice that they’re participating in a clinical trial.”
Another important part of putting patients at the centre of clinical trials is to ensure that sites and sponsors are being inclusive. Another panel spoke about the inclusion of the LGBTQ+ community in clinical research, saying how improvements can be made by changing the language used about gender, amending protocols and eligibility criteria and making sites more inclusive for those in the community.

Shir Netanel, associate director of patient advocacy and clinical trial advocacy for Janssen, highlighted the changes being made in trial protocol, including a change of language, such as calling those enrolled on the trial ‘patients with prostate cancer’. The panel went on to discuss the Healthcare Equality Index which shows which sites are inclusive of the community.
At the end of the panel, Netanal, who is a member of the LGBTQ+ community, comments: “Any opportunity to advocate, going to FDA meetings, say LGBTQ+ data, talk about this in the industry, there is so much more power when it is not just members of the community talking about this.”
Rare paediatric trials can not only be difficult to conduct for those in the industry but can take a big toll on patients and their families. Erin O’Boyle from Rezolute spoke about the importance of not following the status quo in these trials, adding that it is vital to be collaborative, communicative, and cooperative with all partners.

“Nothing is status quo in any rare disease study but bring paediatric into that and that is even more so,” says O’Boyle.
O’Boyle added that transparency is the key for both sites and patients to allow trust with parents to enrol their children on these studies, putting the patient at the heart of the planning process.
This includes simple touches such as ensuring that if patients and families are travelling they have a fridge in their room or water available. It is also important for both sites and patients to have one point of contact to keep consistency.
AI and ML both big talking points at SCOPE
The second plenary session looked at AI and ML and their use in not only data processing but writing protocols and determining eligibility.
The conversation included questions about whether some level of human interaction should remain, with everyone agreeing that it should, as well as inherent bias as a result of the provided data.
As a result, the panel went on to speak about when AI should be monitored by a human or whether a human should continue to undertake tasks.
Neil Garrett, head of regulatory medical writing at Janssen, spoke about writing papers and that AI could be assistive with producing the first draft and then allowing the author to go from there.

“From a medical writing perspective of protocol development, there’s a huge number of AI tools that are available that can help evaluate patient feasibility,” says Garrett. “But you’re not going to allow the AI to make that decision on what the protocol is, you’re still going to need that human intervention.”
AI remained a common theme through the plenary sessions, with a fireside chat with two staff members of the US Food and Drug Administration (FDA) speaking about the use of AI and ML in clinical trials and drug development.
Kevin Bugin, deputy director of operations for the Center for Drug Evaluation and Research (CDER) and Marsha Samson, who works in the CDER office focusing on AI and machine learning (ML), both spoke about the FDA’s draft guidance on AI and ML in clinical trials and how the regulator is working with those in the industry to improve understanding.
Samson said that the FDA has released draft guidance and continues to take feedback from sponsors, biotech CROs and sites about how the guidance can further be improved.
Sustainability in the spotlight
One of the most prevalent topics of discussion was how trials can be more sustainable. At an opening session on Sunday 11 February, Jason Lainer, global programme leader and director at Johnson & Johnson; and David Lumby, executive director of executive director of environment, health and safety (EHS) at PPD, a subsidiary of Thermo Fisher Scientific, explained what their companies are doing.
Lainer said that the company is reducing its carbon footprint, including using the Kits4Life scheme which repurposes supplies, lab kits, and equipment to countries in need around the world.
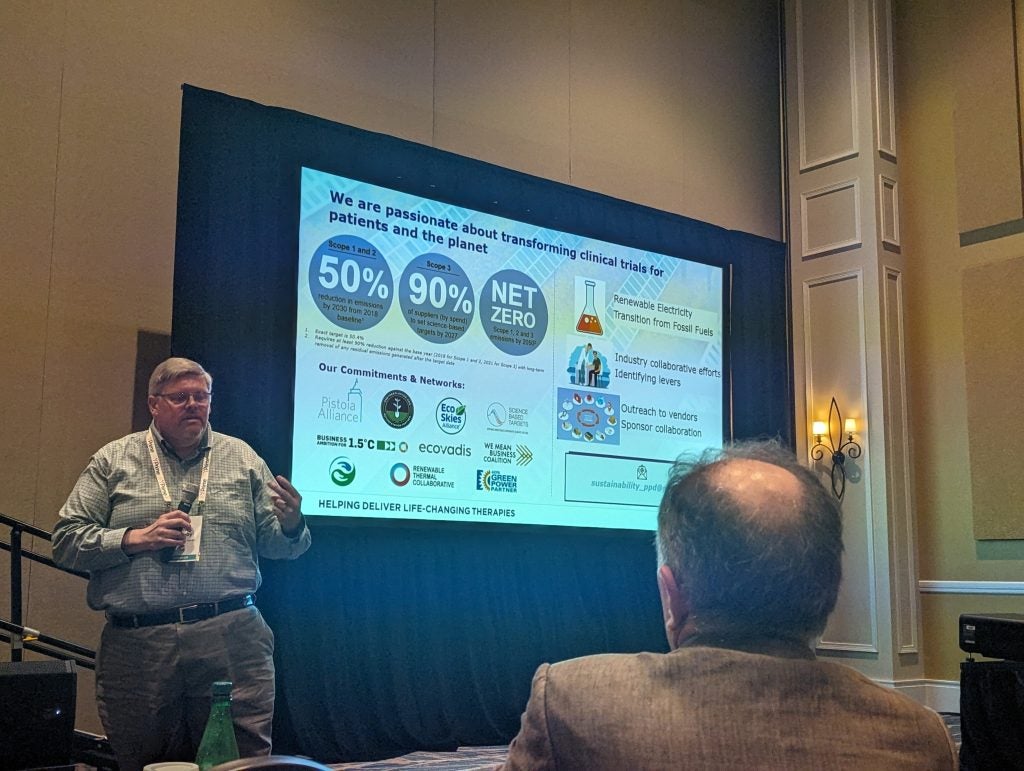
Meanwhile, Lumby spoke about the efforts that they are making to not only reduce scope one and two emissions but also work with partners to reduce scope three emissions, with an ambitious plan for Thermo Fisher to become net zero in all three areas by 2050.
Michael Cohen, senior director of environmental sustainability at Thermo Fisher Scientific, said that total clinical trial activity can produce between 37.6 to 100 megatons of fuel – equivalent to the annual use of the whole of Sweden.
Cohen spoke about improving the sustainability of clinical research associate (CRA) visits by using airlines with more environmentally friendly fuel where possible and reducing multiple visits to the same area by visiting several sites per trip.
Cohen held another session focusing on making patient travel more sustainable. Cohen said: “Patients are travelling far distances to join Phase I, II and III studies. If we think about this holistically, clinical trials, do they matter from an environmental perspective?”
Technology advancing endpoints
There were also advances in digital markers discussed at the conference, including an AI model that assesses a patient’s face and voice to detect signs of Alzheimer’s disease.
The technology, produced by David Suendermann-Oeft at Modality AI uses an AI person called Tina who speaks to patients with mild cognitive impairment and detects markers in patient’s facial movements and voice to detect the state of their decline.
Suendermann-Oeft said that this is something he can detect when observing a patient very quickly which is what inspired him to develop the technology. It is currently being used in Alzheimer’s disease clinical trials with good patient feedback on the software.

There was also an interim announcement by Melissa Ceruolo, vice president of engineering and biomarker analytics for Medidata, who spoke about advancements in the space of the six-minute walk test (6MWT), a standard endpoint used in a variety of respiratory trials.
Cerulo discussed how this could be advanced and how the new metric allows the test to be reduced to 30ft instead of 90ft so a patient can conduct this test at home.
The technology monitors the heart rate so that average heartbeats per metre can be calculated to see not only the distance covered but also the stress exerted over that distance. Ceruolo says that this calculation will better represent a patient’s ability on the test.





