Zybrestat is a vascular disrupting agent (VDA) indicated for the treatment of anaplastic thyroid cancer and, potentially, other solid tumours. The drug is the product of research by Oxigene, a biotechnology company focusing on small-molecule therapeutics to treat cancer and ophthalmic diseases.
A member of a new class of anti-cancer drugs, Zybrestat is in Phase II/III trials under a special protocol assessment agreement with the US Food and Drug Administartion (FDA).
Zybrestat has been granted orphan drug status by the FDA and the European Medicines Agency for the treatment of anaplastic thyroid cancer.
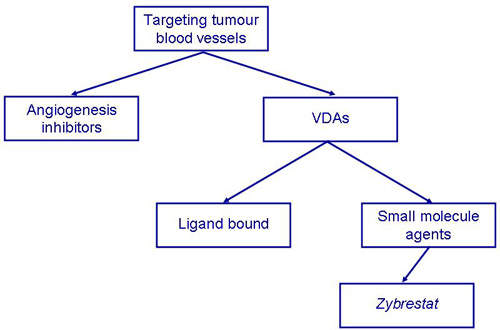
VDAs target established tumour vasculature
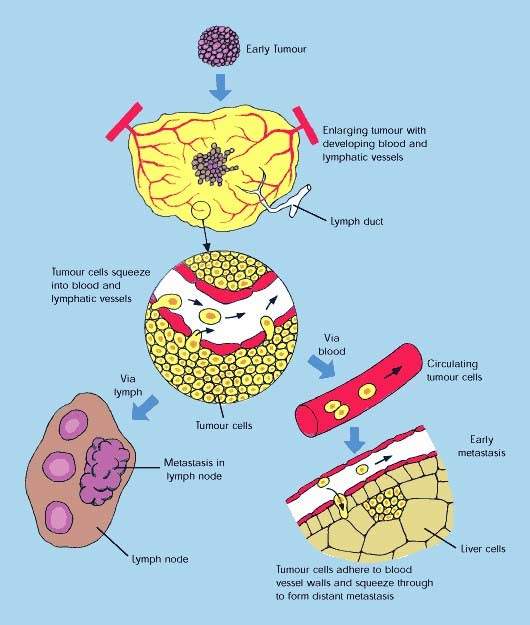
The concept of attacking tumours by cutting off their blood supply was first described in the early 1970s and subsequently led to the development of a new class of anti-cancer drugs called angiogenesis inhibitors. These drugs, which are now in clinical use, work by preventing tumours from developing a blood supply, a pre-requisite for tumour growth and metastasis (tumour spreading).
VDAs differ from angiogenesis inhibitors in that they are designed to attack the established blood vessel network within a tumour rather than preventing the growth of new blood vessels from the viable tumour rim.
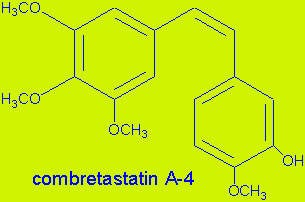
Zybrestat (combretastatin A4 phosphate) is a prodrug that is converted to combretastatin inside the endothelial cells that line blood vessels. Combretastatin has a dual-mode of action, targeting both VE-cadherin, a junction protein that is important for endothelial cell survival, and the associated beta-catenin/AKT signalling pathway.
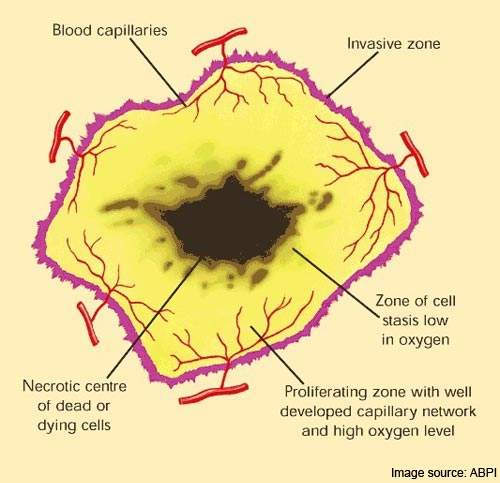
Once activated within the endothelial cell, it causes rapid collapse and necrosis of the tumour’s vascular structure.
As a reversible tubulin depolymerizing agent, combretastatin also causes tumour-associated endothelial cells to change from a flat to a round shape. This has the effect of plugging the blood vessels so depriving the tumour of the oxygen and nutrients it needs to survive.
Because the endothelial cells of tumours are immature they are much more sensitive to the effects of combretastatin than the endothelial cells of normal tissue.
Zybrestat shows evidence of anti-tumour activity
Data from a phase II study, in which the clinical effects of Zybrestat in combination with paclitaxel and carboplatin were studied in 13 patients with advanced malignancies including anaplastic thyroid cancer, suggest that Zybrestat possesses potent
anti-tumour activity.
Patients in this study received one of two dose regimens of Zybrestat, both of which reduced tumour blood flow as measured by dynamic contrast-enhanced MRI.
The most marked reductions in tumour blood flow (70%) were seen in the patients with anaplastic thyroid cancer.
In September 2010, Oxigene announced positive results from the ongoing Phase II/III Fosbretabulin in Anaplastic Cancer of the Thyroid (FACT) study, which is investigating the drug’s effectiveness in combination with paclitaxel and carboplatin, compared to treatment with paclitaxel and carboplatin alone.
Around 180 patients were initially expected to be enrolled, but only 80 patients were eventually taken on. Results indicate that the median overall survival improved by 5.1 months in patients who were administered Zybrestat, while it improved by 4.1 months in the case of patients who received chemotherapy.
Combining VDAs with angiogenesis inhibitors
The potential to use VDAs in combination with angiogenic inhibitors is a concept that is attracting considerable interest among scientists. Targeting different aspects of a tumour’s blood supply, sequential use of VDAs and angiogenic inhibitors could lead to massive tumour necrosis and destruction.
While a VDA such as Zybrestat could be used to destroy the established blood supply feeding the tumour, the subsequent addition of an angiogenic inhibitor could prevent the regrowth of blood vessels (neovascularisation) which allows a tumour to survive and proliferate following initial therapy. Preventing the regrowth of blood vessels from the viable tumour rim could help stop tumours from spreading.
marketing commentary
Oxigene’s Zybrestat is under development for the treatment of anaplastic thyroid cancer, a highly aggressive primary thyroid malignancy for which there are no approved treatments.
Currently, newly diagnosed patients have a median life expectancy of about three months. Although relatively rare, it represents a disease of significant unmet clinical need. VDAs, of which Zybrestat is one of several in development, may have the potential to treat difficult malignancies such as anaplastic thyroid cancer as well as other solid tumours when used in conjunction with established cancer drugs.