ASA404 is a vascular disrupting agent (VDA) that was being developed by Antisoma and Novartis as a treatment for a range of solid tumours. Originally discovered by scientists in New Zealand, ASA404 was licensed to Antisoma in 2001. Novartis subsequently acquired worldwide rights to ASA404 in 2007.
On the back of encouraging Phase II trials results in advanced lung cancer, ASA404 was scheduled to enter Phase III trials in patients with non-small cell lung cancer.
However, in March 2010, Antisoma announced that ASA404 had failed the Phase III ATTRACT-1 study. Antisoma stopped the trial after an interim analysis, which indicated that ASA404 did not demonstrate any significant survival benefits for patients suffering from untreated non-small cell lung cancer.
Another Phase III trial, ATTRACT-2, was still being carried at the time of termination of the first study. In November 2010, however, following an interim analysis the ATTRACT-2 study was also terminated. The analysis indicated that the drug was unlikely to provide any significant benefits as a second-line treatment for non-small cell lung cancer.
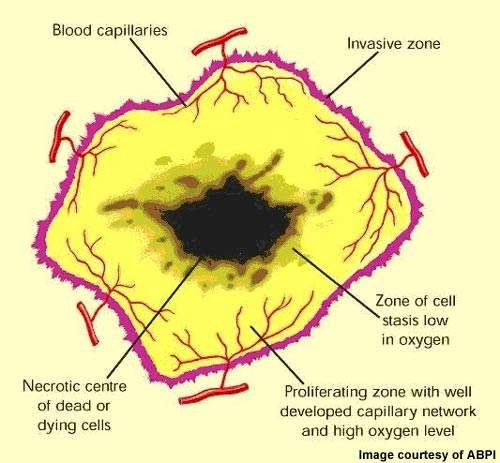
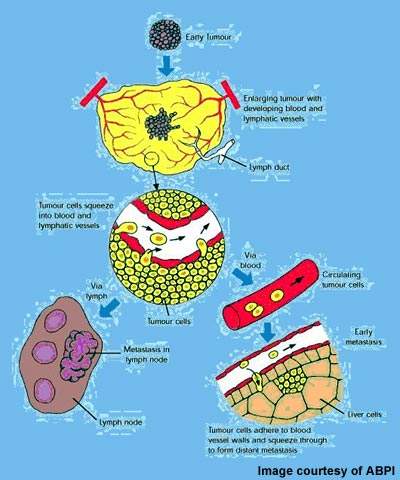
VDAs target established tumour vasculature
The concept of attacking tumours by cutting off their blood supply was first described in the early 1970s and subsequently led to the development of a new class of anticancer drugs called angiogenesis inhibitors.
These drugs, which are now in clinical use, prevent tumours from developing a blood supply – a prerequisite for tumour growth and metastasis.
VDAs differ from angiogenesis inhibitors in that they are designed to attack the established blood vessel network within a tumour rather than preventing the growth of new blood vessels from the viable tumour rim.
Antisoma’s ASA404 (5,6-dimethylxanthenone-4-acetic acid) is a small molecule VDA. In preclinical studies it demonstrated additive or supra-additive anti-tumour effects when combined with standard cytotoxic agents, most notably taxanes.
Efficacy in non-small cell lung cancer
Non-small cell lung cancer is an aggressive disease that has proved notoriously difficult to treat. Despite the availability of new, targeted drugs for lung cancer, prognosis for many patients remains poor.
Phase II trials in non-small cell lung cancer patients suggested that the addition of ASA404 to conventional treatment with carboplatin and paclitaxel significantly enhances overall clinical effectiveness. Indeed, the companies believe that the addition of ASA404 to chemotherapy has produced one of the largest increases in median survival ever reported in advanced lung cancer patients.
Results from the randomised Phase II trial, in which patients received a 1,200mg/m² dose of ASA404 in combination with chemotherapy, showed median survival was 14.0 months in the ASA404 treatment arm compared with 8.8 months in those receiving chemotherapy alone.
A subsequent open-label extension to the randomised trial, in which a higher dose of 1,800mg/m² ASA404 was used in combination with carboplatin and paclitaxel, confirmed these findings. Median survival was 14.9 months and median time to tumour progression 5.5 months, while the overall tumour response rate was 37.9%.
In April 2008, Novartis announced the start of Phase III clinical trial ATTRACT-1 (Antivascular Targeted Therapy: Researching ASA404 in Cancer Treatment) for ASA404.
The trial, conducted at more than 20 sites in 200 countries, was a randomised, double-blind, placebo-controlled, study of ASA404 with paclitaxel and carboplatin. It examined ASA404 as a first-line treatment for non-small cell lung cancer, and covered squamous and non-squamous histology.
In January 2009, Novartis launched Phase III clinical trial ATTRACT-2 for ASA404, which examined ASA404 as a second-line treatment for non-small cell lung cancer.
The trial recruited 900 patients who had undergone chemotherapy treatment alone and patients who received chemotherapy along with bevacizumab or cetuximab as a first-line treatment.
Potential in other solid tumours
Although early clinical trials in patients with ovarian cancer failed to produce the positive results seen with ASA404 in the lung cancer trials, encouraging results have been reported in prostate cancer patients.
Improvements over chemotherapy alone were observed in a 74-patient open-label study, in which chemotherapy-naive patients were randomised to ASA404 plus docetaxel or docetaxel alone (standard therapy).
In this population of patients with progressive, hormone-refractory metastatic prostate cancer, treatment with ASA404 plus docetaxel was associated with a higher PSA response compared with standard therapy alone (57% versus 35%).
In addition, fewer patients in the combination treatment arm showed PSA progression.
Further trials are needed to confirm these early indications of efficacy in prostate cancer.
Marketing commentary
VDAs, none of which are yet approved as a cancer treatment, are seen as a potentially attractive new class of anti-cancer agents, able to potentiate the effectiveness of existing anticancer drugs.