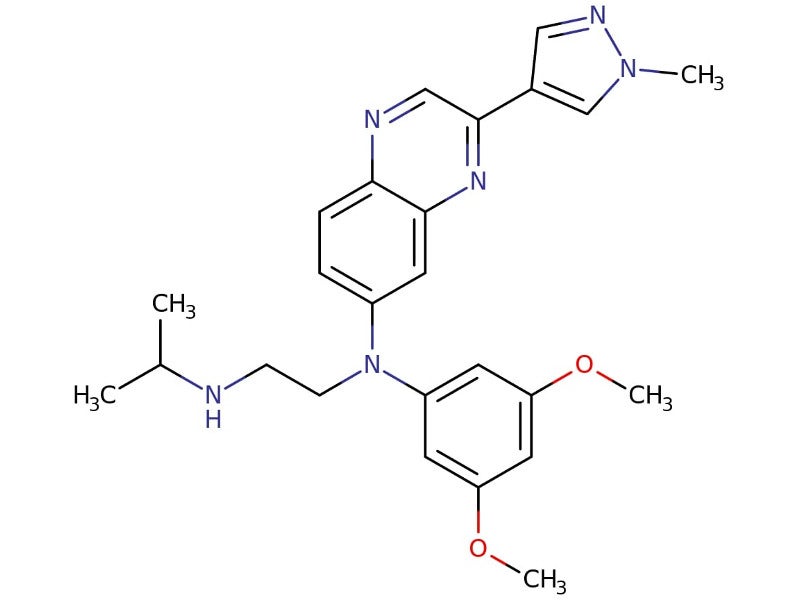
BALVERSA® (erdafitinib) is a fibroblast growth factor receptor (FGFR) kinase inhibitor indicated for the treatment of patients with locally advanced or metastatic urothelial carcinoma (mUC) who have susceptible FGFR3 genetic alterations and whose disease has progressed following at least one previous lines of systemic treatment.
It is the first and only targeted treatment approved for treating mUC with FGFR mutations.
The drug was developed by Astex Pharmaceuticals and Johnson & Johnson (J&J) Innovative Medicine, previously known as Janssen Pharmaceuticals, under an agreement signed in June 2008.
Janssen-Cilag International, a subsidiary of J&J, holds the marketing rights of BALVERSA in Europe.
The US Food and Drug Administration (FDA) approved the companion diagnostic device QIAGEN therascreen® to determine a patient’s eligibility for BALVERSA.
Developed by QIAGEN Manchester, the polymerase chain reaction (PCR) kit is one of the first FDA-approved PCR-based companion diagnostics to detect FGFR mutations.
BALVERSA is available as a round, biconvex, film-coated tablet in 3mg, 4mg or 5mg strengths. The 3mg tablet is yellow in colour, while the 4mg and 5mg tablets are orange and brown, respectively.
Regulatory approvals for BALVERSA
BALVERSA received the FDA’s accelerated approval in April 2019, which later got converted into full approval in January 2024.
BALVERSA also received approval from the European Commission in August 2024. The drug received breakthrough therapy designation from the FDA in March 2018.
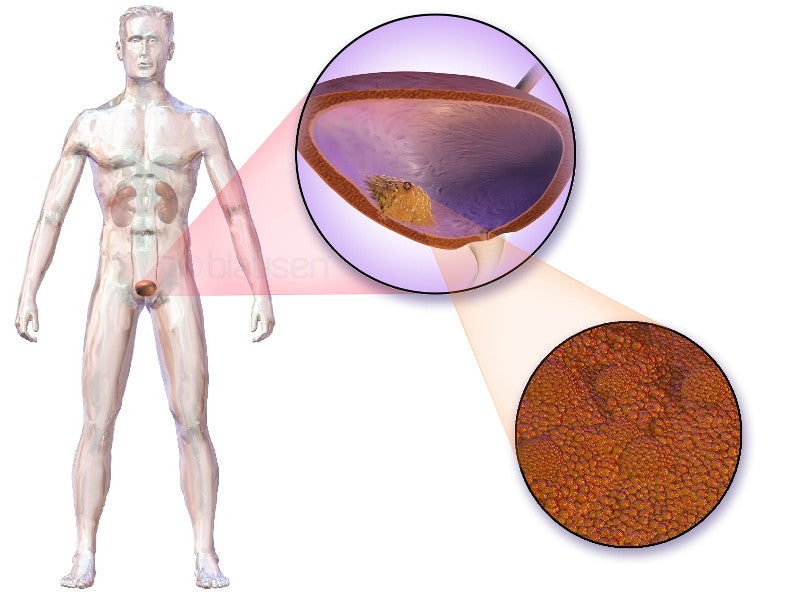
Urothelial carcinoma causes and symptoms
Urothelial carcinoma, also known as transitional cell carcinoma, originates in the bladder’s innermost lining, known as urothelium.
When the tumour spreads to the bladder walls or lymph nodes, it is called locally advanced carcinoma. Conversely, when it extends beyond the bladder to other body parts, the condition is classified as metastatic.
This is among the most prevalent types of bladder cancer and ranks as the sixth most common cancer in the US. It accounts for more than 90% of bladder cancer cases.
Notably, one in five patients with advanced urothelial carcinoma harbours a mutation in the FGFR gene, which drives increased growth and survival of tumour cells. The five-year survival rate for patients with stage IV metastatic bladder cancer stands at 8%.
BALVERSA’s mechanism of action
Erdafitinib acts as a pan-FGFR tyrosine kinase inhibitor that blocks FGFR kinases, essential for gene functioning.
Erdafitinib binds and inhibits the enzymatic activity, phosphorylation and signalling of FGFR genes, leading to the death of malignant cells.
Clinical trials on BALVERSA
The FDA’s approval of the drug was based on the positive results of the THOR clinical trial.
THOR is a Phase III, open-label, multicentre trial assessing the efficacy and safety of BALVERSA in patients with locally advanced or mUC harbouring susceptible FGFR3 genetic mutations, following progression on or after at least one previous systemic treatment.
The study compared BALVERSA in two cohorts with the patients randomised in a 1:1 ratio. The primary endpoint was overall survival (OS).
In cohort 1, patients were administered BALVERSA or the investigator’s choice of chemotherapy (docetaxel or vinflunine) after at least one line of treatment inclusive of an anti-programmed death ligand-1 (PD-(L)1) agent. The median follow-up period was 15.9 months.
The median OS for cohort 1 was 12.1 months with BALVERSA compared to 7.8 months with chemotherapy, demonstrating a more extended OS with BALVERSA treatment.
In Cohort 2, BALVERSA was evaluated against pembrolizumab following one previous treatment excluding an anti-PD-(L)1 agent. The median follow-up duration is 33 months. The median OS with BALVERSA and chemotherapy was 10.9 and 11.1 months, respectively.
The study demonstrated a 36% reduction in the risk of death with BALVERSA compared to chemotherapy in patients.
The most common adverse events reported in the patients were eye diseases, an increase in phosphate and calcium, a decrease in sodium, magnesium, phosphate and albumin, stomatitis, fatigue, diarrhoea, onycholysis, dry skin, a decrease in haemoglobin count, impaired sense of taste, abdominal pain nausea and musculoskeletal pain.