Bifeprunox mesilate was a novel atypical antipsychotic agent under development by Solvay Pharmaceuticals as a treatment for schizophrenia and potentially conditions such as bipolar disorder. In July 2009, the company announced that all development activities related to the drug would be halted.
Solvay and US marketing partner Wyeth filed for regulatory approval for bifeprunox as a treatment for schizophrenia with the US Food and Drug Administration (FDA) in October 2006. However, the regulator had concerns about the efficacy and safety of the drug, and rejected the application in August 2007. Wyeth subsequently decided to pull out of the agreement and all US rights to bifeprunox have now been returned to Solvay.
In July 2009, Solvay and its European marketing partner, Lundbeck, announced that all development activities related to bifeprunox will be ceased.
The decision was taken following the interim analysis of pooled data conducted to extend an ongoing phase III clinical trial. The data indicated that bifeprunox did not have any impact on the stabilisation of non-acute patients suffering from schizophrenia. Although the drug demonstrated some efficacy, the companies felt that it was not enough to pursue marketing approval.
Dopamine partial agonists represent a new class of atypicals
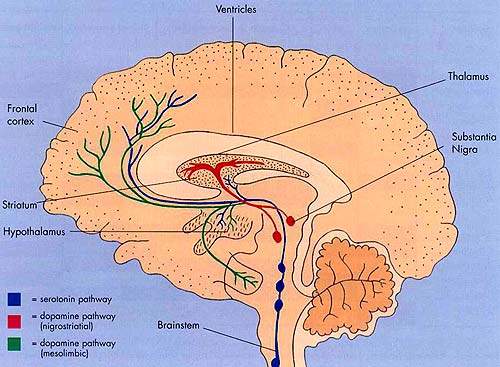
The first generation of atypical antipsychotic drugs, which entered clinical practice over a decade ago, share the common property of dopamine D2 receptor antagonism.
This mode of action is believed to be the primary pharmacological mechanism by which antipsychotic drugs ameliorate the positive, psychotic symptoms of schizophrenia.
Bifeprunox differs from first-generation atypical antipsychotics in that it acts as a partial D2 agonist. This property is shared by aripiprazole, a drug already marketed in Europe and the US for treatment of schizophrenia.
In contrast to D2 receptor antagonism, partial D2 agonism is believed to simultaneously decrease dopamine activity in overactive dopamine systems while increasing its activity in regions of the brain where dopaminergic activity is too low.
By blocking overstimulated receptors and stimulating underactive ones, partial D2 agonists act as dopamine stabilisers.
In common with aripiprazole, bifeprunox also acts as a serotonin, 5-HT1A agonist. This property may contribute to efficacy against the negative symptoms of schizophrenia and reduce the likelihood of extrapyramidal symptoms.
This new class of atypical antipsychotic drugs, sometimes described as next-generation antipsychotics, is seen as an attractive treatment option for schizophrenia and, potentially, the acute manic phase of bipolar disorder.
Increasing treatment options
Despite an expanding range of antipsychotic drugs, which have greatly improved treatment prospects for patients with schizophrenia, there remain significant treatment gaps. New antipsychotic drugs are needed with broader efficacy to treat patients unresponsive to current agents.
Although atypical antipsychotics offer improved safety and tolerability over conventional typical antipsychotics, especially with respect to extrapyramidal symptoms, drug safety is still an issue. Significant weight gain leading to metabolic disturbances (such as diabetes and heart disease), sexual dysfunction, hyperprolactinaemia, and cardiotoxicity are unwelcome side effects associated with some currently used atypical drugs.
Poor tolerability leads to poor treatment compliance and risk of relapse. Thus, there is a need for antipsychotics with improved safety and tolerability to improve compliance and outcome.
A placebo-controlled, dose-finding Phase II trial in patients with schizophrenia showed that bifeprunox was both efficacious and well tolerated. Importantly, treatment with bifeprunox did not appear to cause some of the side effects seen with other atypicals agents in routine use.
In addition to a low incidence of extrapyramidal symptoms, similar to placebo, other benefits that may arise from clinical use of bifeprunox include:
- no weight gain
- no increase in prolactin levels
- no adverse effect on blood lipids
- no adverse effects on blood glucose levels
- no QTc prolongation.
Wyeth ends partnership with Solvay
Although Lundbeck secured worldwide development and marketing rights to bifeprunox outside Canada, Japan, Mexico and the US, Solvay had forged a partnership with Wyeth for the key US market.
The co-development and marketing agreement between the two companies, signed in April 2004, focused on four of Solvay’s investigational antipsychotic drugs: bifeprunox, SLV-310, SLV-313 and SLV-314.
Wyeth’s decision to end its partnership with Solvay arose in part from the FDA decision to reject bifeprunox and in part from the belief that there was insufficient commercial value in the product for the two companies to share. All development and commercialisation rights in the US for bifeprunox, as well as global rights for the other compounds, were returned to Solvay.
Marketing commentary
Affecting around 1% of the general population, schizophrenia is a severe and debilitating mental illness. Antipsychotic drugs are the cornerstone of the management of schizophrenia and are designed to address the core symptoms of the illness, which include positive, negative, cognitive and affective symptoms.
Bifeprunox was of one four antipsychotic drugs in advanced-stage development for treatment of schizophrenia. Analysts had believed that of the four, bifeprunox was the most likely to succeed, given its promising efficacy and side-effect profile.
However, this fell in doubt given its failure to secure FDA approval, which stemmed largely from the drug’s inability to perform as well or better than existing antipsychotic drugs. Physicians were unlikely to switch patients from a drug that is working to one that may not work as well.
In the US, six atypical agents currently compete for a share of the antipsychotic sector, while in Europe seven atypical agents are now in routine clinical use.