Amgen’s prolia (denosumab) is a biological therapy developed for the prevention and treatment of osteoporosis and other disorders characterised by bone loss. These include postmenopausal osteoporosis, rheumatoid arthritis, and cancer treatment-induced bone loss.
In May 2010, Amgen was granted marketing approval for prolia by the European Commission (EC) to treat osteoporosis in postmenopausal women and for treating bone loss linked with hormone ablation in men suffering from prostate cancer.
In June 2010, Amgen also received FDA approval for prolia for treating postmenopausal women with osteoporosis.
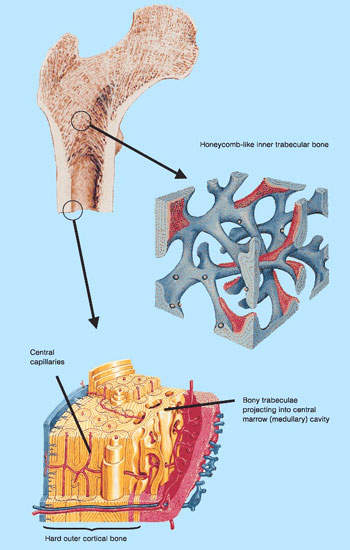
Osteoporosis and other bone loss conditions
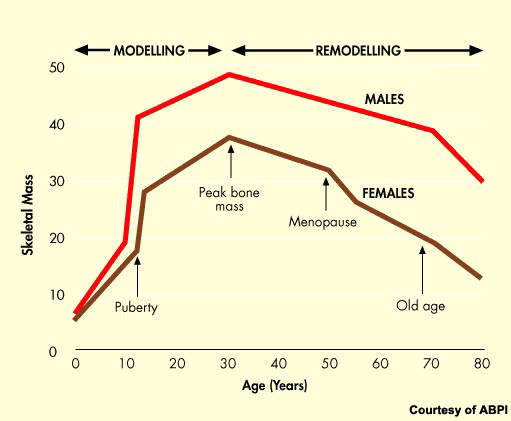
Osteoporosis is a serious health problem that affects millions of post-menopausal women. The progressive loss of bone density that follows the menopause predisposes women to greatly increased risk of bone fractures.
As the proportion of elderly people in the population of industrialised nations continues to grow, so the problem will grow and place an increasingly heavy burden on healthcare budgets.
Data from the US show that currently over 10 million people have osteoporosis with a further 34 million estimated to have low bone mass, a risk factor for osteoporosis.
Bone loss is associated with a number of other disorders including multiple myeloma, bone metastases and rheumatoid arthritis. It can also occur as a consequence of hormone-ablative therapy for breast or prostate cancer.
Prolia targets RANK ligand
By targeting RANK ligand, prolia represents an entirely new approach to the prevention and treatment of bone loss. RANK ligand is a protein that plays a pivotal role in the process during which bone is formed and resorbed. RANK ligand is the primary mediator of osteoclast formation, cells responsible for bone removal.
In diseases associated with bone loss there is an imbalance between RANK ligand and osteoprotegerin, a protein that counteracts the action of RANK ligand. By blocking the effects of RANK ligand, prolia effectively mimics the bone-protecting effects of osteoprotegerin.
In preclinical models of bone loss, inhibition of RANK ligand proved an effective approach to improving cortical and trabecular bone density, volume, and strength.
Clinical trials
The clinical effectiveness of prolia was investigated in a series of clinical trials including six phase III and two phase II trials in post-menopausal osteoporosis.
Amgen conducted a two-year, multicentre, open label phase III clinical trial on Prolia, named the Denosumab Adherence Preference and Satisfaction Study (DAPS). The study enrolled 250 postmenopausal women. The patients were randomised to receive prolia for six months over a weekly dose of alendronate.
The study results, announced in March 2011, showed that about 92.4% of the patients preferred prolia over alendronate.
The FDA approval for prolia was based on a pivotal three-year phase III clinical study called FREEDOM (Fracture REduction Evaluation of Denosumab in Osteoporosis every six Months). The study enrolled 7,808 postmenopausal women with osteoporosis, who were administered with prolia as a 60mg subcutaneous injection every six months.
Results showed that the patients treated with prolia experienced 68% reduction in vertebral fractures, 40% reduction in hip fractures and 20% reduction in non-vertebral fractures. The drug also increased the bone density significantly.
In February 2010, Amgen announced the results of a pivotal phase III, head-to-head trial evaluating 120mg Prolia for every four weeks with 4mg zometa (zoledronic acid). The study enrolled 1,901 men who had advanced prostate cancer with bone metastases.
The results showed that prolia demonstrated superiority over zometa in patients with bone metastases. Prolia met all primary and secondary endpoints.
Amgen conducted a pivotal phase III clinical trial on Prolia named HALT (Hormone Ablation Bone Loss Trial). The study enrolled 1,468 patients and evaluated the change from baseline in lumbar spine BMD.
The results of the study showed that the relative risk of suffering a new vertebral fracture within 12 months in prolia-administered patients was lowered by 62% compared with placebo at 36 months.
In a double-blind, head-to-head study with alendronate in post-menopausal women with low bone mineral density (BMD), prolia achieved significantly greater BMD gains at the total hip, hip trochanter and distal radius. BMD improvement at the total hip was approximately 40% greater in the prolia treatment arm compared with those receiving alendronate (primary endpoint).
Twice-yearly injections of prolia also achieved significant increases in BMD in a pivotal 332-patient study, in which it was compared with placebo.
At the end of the two-year study period, prolia-treated patients had a significantly greater increase in lumbar spine BMD compared with placebo recipients (6.5 vs -0.6%; p<0.001).
Similarly significant differences were observed between active treatment and placebo with respect to BMD at the total hip (3.4 vs -1.1%; p <0.0001), wrist (1.4 vs -2.1%; p<0.0001) and total body (2.4 vs -1.4%; p<0.0001).
Treatment with prolia appeared well tolerated by patients enrolled in all the clinical trials.
Marketing commentary
The market for treatments to address bone loss in osteoporosis and other conditions is large and growing. Until recently, hormone replacement therapy (HRT) was considered the mainstay of prevention and treatment of osteoporosis.
However, concerns about the safety of HRT in long-term use suggest that increasingly it will be restricted to short-term treatment of climacteric symptoms. This change in treatment practice will see greater use of alternative therapies such as bisphosphonates and selective estrogen receptor modulators (SERMS) among others.
Although the range of treatments for osteoporosis is increasing, the need for safe and effective long-term medications for prevention and treatment of bone loss remains. RANK ligand inhibitors represent an entirely new class of agents for osteoporosis and other bone loss conditions.