Lixiana (edoxaban) is an oral anticoagulant developed by Daiichi Sankyo, a pharmaceutical company based in Japan. It is being developed for a range of indications, including atrial fibrillation, venous thromboembolism and arterial thromboembolism.
The drug, also known as DU-176b, works by inhibiting an enzyme involved in blood coagulation called factor Xa. By inhibiting this factor, blood clots are less likely to form and the chances of thromboembolism are reduced.
Sankyo submitted a new drug application for edoxaban to Japan’s Ministry of Health, Labour and Welfare on 6 April 2010, seeking approval for it to be used for the prevention of venous thromboembolism in patients who have undergone orthopaedic surgery.
On 22 April 2011, Sankyo received the marketing approval for Lixiana from Japan’s Ministry of Health and Labour Welfare.
Global Phase III studies for both atrial fibrillation and venous thromboembolism arer under way.
Thrombosis and thromboembolism
Thrombosis is the process of a blood clot (thrombus) forming in a blood vessel. Thromboembolism is the term used to collectively describe the formation of a clot, it breaking loose and it blocking another blood vessel somewhere else in the body.
Atrial fibrillation – a common cardiac arrhythmia – can sometimes cause blood not to be completely pumped out the heart. This blood can potentially form into clots, which can cause a stroke if they break off and block a blood vessel in the brain. It is estimated that people suffering from atrial fibrillation are five times more likely to suffer a stroke.
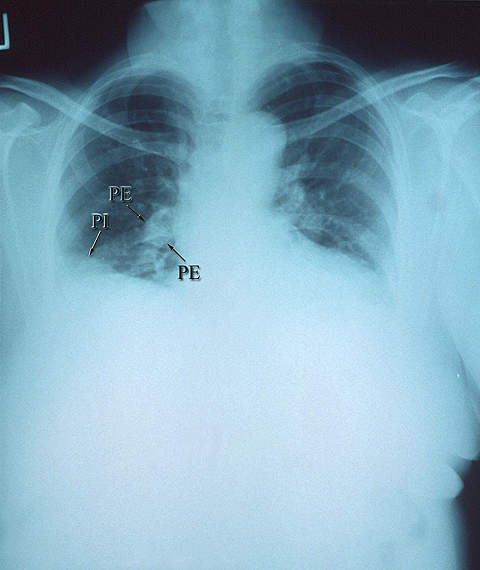
A deep vein thrombosis is a clot that forms in one of the deep veins, such as in the leg or pelvis. When one of these clots caused a blockage in a blood vessel in the lungs, it is known as a pulmonary embolism. Arterial thromboembolisms occur when a clot blocks an artery.
Common treatments for thromboembolisms include anticoagulant drugs and compression stockings. In extreme cases, surgery may be performed.
In the US, there are an estimated 900,000 cases of venous thromboembolism each year, and around 300,000 deaths are linked to the condition. There are an estimated 750,000 cases in France, Germany, Italy, Spain, Sweden and the UK combined, with 370,000 related deaths in these countries.
Clinical trials of edoxaban
Edoxaban has demonstrated a safety and efficacy profile comparable to warfarin (another anticoagulant) in Phase I and Phase II clinical trials.
In Phase IIb, three randomised, double-blinded studies of edoxaban were conducted for atrial fibrillation: the first in the US and Europe with 1,146 patients; the second in Japan with 536 patients; and the third in Asia with 235 patients. Each ran for a period of three months, and no clinically relevant events or increases in bleeding were reported.
Two Phase III studies for venous thromboembolism were then initiated in March 2009, both in Japan. They were conducted in patients who have undergone total knee replacement (TKR) or total hip replacement (THR). Some 610 THR patients and 716 TKR patients were recruited for the two randomised, double-blind studies.
The TKR study was a randomised, double-blind trial that compared edoxaban with enoxaparin sodium. Results from the study were announced in December 2009 and indicated that there was no major difference between the two drugs.
The THR study was a multicentre, double-blind, and centrally-randomised trial. The results indicated that edoxaban had higher efficacy compared to enoxaparin sodium after THR. Deep vein thrombosis was observed in 2.4% of patients who were administered with edoxaban and in 6.9% of patients treated with enoxaparin sodium.
Daiichi Sankyo filed the new drug application for edoxaban in Japan following completion of these two trials. Global development of edoxaban involves two separate Phase III trials, both of which are randomised and double-blind.
The first, called ENGAGE AF-TIMI 48, is a multinational, randomised and double-blind study was initiated towards the end of 2009. It will test the safety and efficacy of the drug in atrial fibrillation patients in comparison with warfarin. About 21,000 patients have been recruited, and will take part at one of 1,400 centres across 46 countries.
The second trial, called Hokusai, is a single, double-blind, randomised and multinational Phase III study announced in February 2010. It will test the drug’s safety and efficacy in reducing recurrent venous thromboembolic complications in the deep vein thrombosis and pulmonary embolism patients. The study recruited 7,500 patients across 450 centres in 40 countries.
Marketing commentary
Oral factor Xa inhibitors represent an emerging treatment option for atrial fibrillation, venous thromboembolism and arterial thromboembolism patients.
Current treatment methodologies lack reliable efficacy and safety profiles. They give rise to safety issues such as bleeding and unpredictable food and drug interactions, and require constant monitoring.
Factor Xa inhibitors such as edoxaban have a better safety profile and do not require constant monitoring.