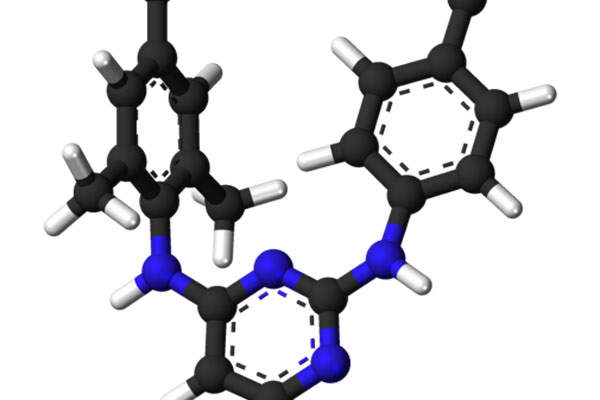
Edurant (rilpivirine) is an antiviral drug indicated for treatment-naive adults with HIV-1 infection. The drug was originally developed by Tibotec Pharmaceuticals, which became part of Janssen Pharmaceutical Company in June 2011 for a greater collaboration in the development of rilpivirine. The company’s name was also changed to Janssen Therapeutics.
The US Food and Drug Administration (FDA) granted marketing approval for Edurant in May 2011. It was approved for use in combination with other antiretroviral agents (ARVs) for the treatment of HIV-1 infection in adults who have previously not taken any HIV treatment.
Janssen obtained the marketing authorisation for Edurant from the European Commission (EC) for the treatment of HIV-1 infection in adult patients with a viral load ≤ 100,000 HIV-1 RNA copies/mL in November 2011.
The drug also received approval in Canada in May 2011. The marketing authorisation applications for the approval of Edurant have been submitted in Japan, Russia and Switzerland.
HIV-1 infection treatment
Related project
Sustiva (Efavirenz) – Treatment for HIV-1 Infected Paediatric Patients, United States of America
Sustiva (efavirenz) is an antiretroviral indicated for treatment of paediatric patients affected with the HIV-1 infection.
Human immunodeficiency virus (HIV) is a lentivirus that delivers a significant amount of genetic information into the DNA of the host cell.
HIV is responsible for causing acquired immune deficiency syndrome (AIDS), which is a deadly disease that damages the immune system.
It is categorised into two types, HIV-1 and HIV-2. HIV-1 is more virulent and infective than HIV-2.
HIV is estimated to affect an estimated 50,000 people in the US every year, according to the estimates by Centre for Disease Control and Prevention. The disease resulted in an around 15,500 deaths in 2010.
Edurant (rilpivirine): mechanism of action
Edurant (rilpivirine) is an antiviral drug that contains non-nucleoside reverse transcriptase inhibitor (NNRTI). The drug works by restraining the HIV-1 replication by non-competitive inhibition of HIV-1 reverse transcriptase.
The drug is available in 25mg dose for oral administration in tablet form.
Clinical trials on Edurant (rilpivirine)
The marketing approval for Edurant in Europe was based on two phase III clinical trials known as Efficacy Comparison in treatment-naive HIV-infected subjects Of TMC278 and efavirenz, or TMC278-C209 (ECHO) and TMC278 against HIV, in a once-daily RegImen Versus Efavirenz, or TMC278-C215 (THRIVE) studies lasting for 48 weeks.
The randomised, double blind, active controlled, global Phase 3 clinical trials evaluated the efficacy and safety and tolerability of Edurant in comparison with efavirenz. The studies enrolled 1,350 treatment-naive HIV-1 adult patients across 20 countries.
The studies also found that 90.2% of patients in the Edurant arm achieved an undetectable viral load at week 48 compared with 83.6% in the efavirenz arm. The virologic failure (VF) rate was 9.0% in Edurant administered patients when compared with 4.8% in the efavirenz arm.
FDA approval for Edurant was based on based one phase IIb clinical trial and two phase III clinical trials. The phase III clinical trials were known as ECHO and THRIVE studies.
The phase IIb clinical trial was a randomised, active-controlled study which enrolled 368 HIV-1 patients. The study was completed in two parts. The patients were enrolled for 96 weeks open label study. After 96 weeks the patients were randomised to receive Edurant 25mg once daily or efavirenz 600mg once daily, in addition to a background regimen.
The study’s results demonstrated that more than 76% patients who received Edurant 25mg achieved <50 HIV-1 RNA copies/ml when compared 71% achievement in efavirenz-administered patients. The patients who were administered with Edurant 25mg achieved 63% RNA < 50 copies/mL compared with 61% of patients in the efavirenz group at week 192.
The first phase III ECHO clinical trial was a double blind and randomised study, which enrolled 680 patients with HIV-1 infection. The patients were administered with 25mg of Edurant as once daily oral tablet or efavirenz 600mg tablet, both in combination with emtricitabine, plus tenofovir disoproxil fumarate.
In the second phase III clinical trial, THRIVE was also a double blind and randomised study that enrolled 680 patients with HIV-1 infection. Patients received Edurant 25mg dose tablet once daily or efavirenz 600mg tablet once daily. Both the tablets were used in combination with abacavir + lamivudine, or tenofovir disoproxil fumarate + emtricitabine, or zidovudine + lamivudine.
Both ECHO and THRIVE studies met the primary endpoint of achieving an undetectable viral load. 84.3% of patients treated with Edurant 25mg dose achieved undetectable viral load of less than 50 copies/mL at week 48, where as it was 82.3% in efavirenz administered patients.
Edurant marketing
Tibotec Pharmaceuticals retains the rights of marketing Edurant in the US. Janssen holds the rights to market the drug in Europe. The generic version of the drug was licensed to Hetero Drugs, Emcure Pharmaceuticals, Strides ArcoLab and Matrix Laboratories in India. Aspen Pharmacare is licensed in South Africa for the production, marketing and distribution.
Eviplera is a similar HIV-1 drug manufactured by Gilead Sciences International and Tibotec.