Sanofi and Regeneron’s promising results from rheumatoid arthritis study
France-based Sanofi and US-based Regeneron Pharmaceuticals have reported positive results with sarilumab in the first Phase III rheumatoid arthritis registration trial.
In the SARIL-RA-MOBILITY Phase III clinical study, sarilumab treatment plus methotrexate (MTX) improved disease signs and symptoms as well as physical function, and inhibited progression of joint damage in adult patients with active rheumatoid arthritis who were inadequate responders to MTX therapy.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The Phase III clinical study enrolled approximately 1,200 patients. The 52-week study evaluated three groups of patients who received, in combination with methotrexate, either a 200mg or 150mg dose of sarilumab, or placebo.
Boehringer to initiate two new clinical trials for Pradaxa
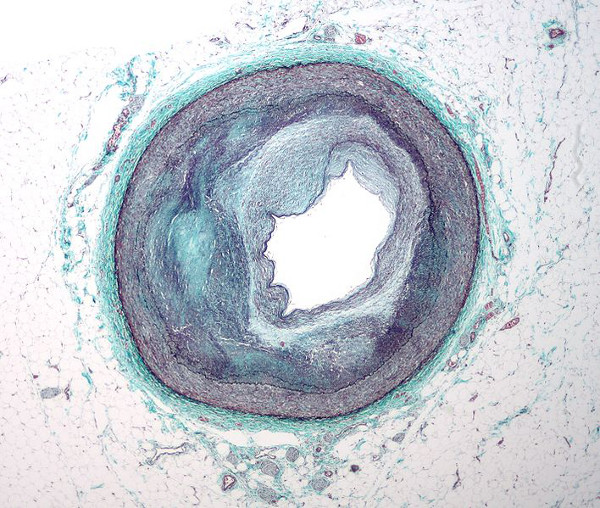
German-based Boehringer Ingelheim is planning to start two large, global clinical trials of Pradaxa (dabigatran etexilate) to assess its efficacy and safety in stroke prevention therapy in two clinically highly relevant conditions.
The first of the two trials is the RE-SPECT ESUS, which is aimed at evaluating the efficacy and safety of Pradaxa for the prevention of recurrent strokes in patients who have suffered an embolic stroke of undetermined source (ESUS).
Embolic strokes typically occur when a blood clot forms somewhere in the body and travel through the bloodstream to the brain.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataGlaxo’s heart drug fails endpoint in Phase III study
UK-based drug manufacturer GlaxoSmithKline (GSK) has released top-line results from the Phase III STABILITY trial, evaluating the efficacy of its investigational Lp-PLA2 inhibitor darapladib in adults patients with chronic coronary heart disease (CHD).
Darapladib was designed to fight heart disease in a new way, but failed to meet its primary endpoint measure in the STABILITY (‘STabilisation of Atherosclerotic plaque By Initiation of darapLadIb TherapY’) trial.
The primary endpoint measure was the time to the first occurrence of any major adverse cardiovascular event (MACE) from composite of myocardial infarction (heart attack), stroke, and cardiovascular death (relative risk reduction of 6%; p=0.199).
GSK and Pfizer to begin Phase I/II study of new combination therapy
GlaxoSmithKline (GSK) and Pfizer have agreed to collaborate on a Phase I/II study to explore the anticancer efficacy and safety of GSK’s trametinib (GSK1120212) combined with Pfizer’s palbociclib (PD-0332991) in patients with advanced/metastatic melanoma.
GSK will be responsible for conducting the dose-escalation, open-label study named ‘Study 200344’, which is designed to determine the recommended combination regimen (RCR) for the combination therapy in patients with melanoma.
The trial will also assess the effect of the combination on tumour biomarkers, safety, and anticancer activity in patients with BRAFV600 wild-type melanoma, including those with NRAS mutations.
GW Pharma initiates Phase Ib/IIa trial to treat glioblastoma multiforme
British biopharmaceutical firm GW Pharmaceuticals has started a Phase Ib/IIa clinical trial for the treatment of recurrent glioblastoma multiforme (GBM).
Twenty patients will be enrolled in the multicentre, two part Phase Ib/IIa trial with an open-label phase which will evaluate safety and tolerability of GW cannabinoids in combination with temozolomide, and a double-blind, randomised, placebo-controlled phase with patients randomised to active or placebo, and with a primary outcome measure of six month progression free survival.
The main objective of the trial is to evaluate the tolerability, safety and pharmacodynamics of a mixture of two principal cannabinoids, THC and CBD in a 1:1 allocation ratio, in combination with temozolomide in patients with recurrent GBM.
Cornerstone begins Phase II study of pancreatic cancer drug
US-based Cornerstone Pharmaceuticals has started a pilot Phase II clinical trial of its lead altered energy metabolism directed (AEMD) drug candidate CPI-613 to treat patients with locally advanced or metastatic pancreatic cancer.
Cornerstone’s CPI-613 is designed to disrupt the altered energy-production pathways in cancer cells.
Generally, pancreatic cancer begins in the tissues of the pancreas, an organ in the abdomen that helps in digestion as well as regulates the metabolism of sugars.
Nanotherapeutics enrols first patient in Phase II cervical cancer trial

US-based Nanotherapeutics has started patient enrolment in a Phase II cervical cancer trial of Triapine, a ribonucleotidereductase inhibitor (RNRi) currently under evaluation in combination with cisplatin and radiation as a radiosensitising chemotherapeutic agent for women with advanced stage cervical/ vaginal cancers.
The double-blinded, randomised, multi-centre trial is sponsored by National Cancer Institute (NCI)’s Division of Cancer Treatment and Diagnosis (DCTD), as part of the clinical trials agreement between NCI and Nanotherapeutics.
A total of 73 women with advanced stage cervical/vaginal cancer will be enrolled in the trial, which will investigate cisplatin and radiation therapy, with or without Triapine.
Amgen reports interim results from Phase III study of melanoma drug
US-based Amgen has reported interim overall survival (OS) results from a Phase III trial assessing the safety and efficacy talimogene laherparepvec (T-VEC) in patients with unresected stage IIIB, IIIC or IV melanoma compared to granulocyte-macrophage colony-stimulating factor (GM-CSF).
As per the predefined interim analysis of the global, randomised, open-label Phase III trial, median OS was 23.3 months in the talimogene laherparepvec arm compared with 19 months in the GM-CSF arm.
According to the company, differences in survival rates were pronounced in the subset of patients with stage IIIB, IIIC or IV M1a disease or who received talimogene laherparepvec as first-line treatment, each comprising about 50% of the trial population.
Medivation, Astellas Pharma begin Phase IV study of prostate cancer therapy

Medivation and Astellas Pharma US, a subsidiary of Tokyo-based Astellas Pharma, have announced the commencement of a Phase IV clinical trial ‘PLATO’.
PLATO is a double-blind, placebo-controlled trial designed to measure the safety and efficacy of continued treatment with enzalutamide plus abiraterone acetate and prednisone when compared to treatment with abiraterone acetate and prednisone alone in patients with chemotherapy-naive metastatic prostate cancer whose disease has progressed following enzalutamide therapy.
The Phase IV study will enrol approximately 500 chemotherapy-naive patients with metastatic castration-resistant prostate cancer.
UK researchers to begin clinical trial of potential HIV cure in 2014
Scientists and clinical researchers from five leading British universities are planning to start a new clinical trial in 2014 to test a possible cure for human immunodeficiency virus (HIV).
The researchers, led by John Frater of Oxford University and Sarah Fidler of Imperial College, London, expect that the trial will demonstrate that a cure is feasible.
The new therapy to be tested in the trial will combine standard antiretroviral drugs with two new weapons including a drug that reactivates dormant HIV and a vaccine that induces the immune system to destroy the infected cells.