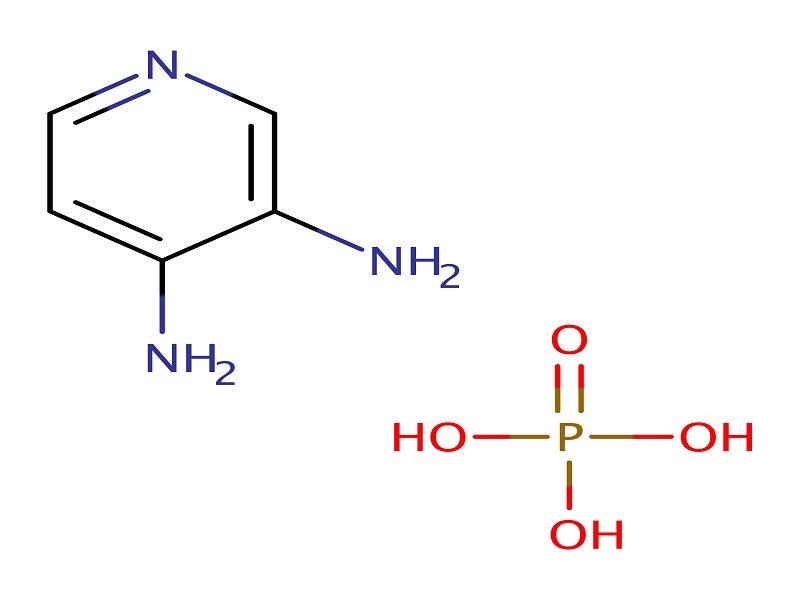
Firdapse® (amifampridine) is one of the first drugs to be approved for the treatment of Lambert-Eaton myasthaenic syndrome (LEMS) in adults.
The drug was developed by BioMarin Pharmaceutical and is marketed by Catalyst Pharmaceutical, which secured the marketing rights to Firdapse in North America for $5m in October 2012. The EU distribution rights are retained by BioMarin.
In December 2009, the European Commission (EC) granted marketing authorisation to Firdapse for the symptomatic treatment of LEMS. The drug also secured orphan drug designation in the EU.
The US Food and Drug Administration (FDA) granted breakthrough therapy designation to the drug in August 2013. Catalyst Pharmaceutical submitted a new drug application (NDA) for Firdapse to the FDA in March 2018, and the application was accepted and granted priority review status in May 2018.
Firdapse was approved by the FDA in November 2018.
Lambert-Eaton myasthaenic syndrome causes and symptoms
LEMS is a rare autoimmune disorder that occurs when an individual’s immune system attacks its own tissues, particularly at the neuromuscular junction. The condition compromises the nerve cells’ ability to transmit signals to the muscle cells, causing muscle weakness.
The disease was named after the neurologists Edward Lambert and Lee Eaton, who first described the disease in the 1950s and 1960s.
The condition affects three in every million individuals worldwide. An estimated 50% to 60% of LEMS patients also suffer from an underlying cancer such as small cell lung cancer.
The most common symptoms of the disease are weakness in the limbs and eye muscles, fatigue, and difficulty talking and swallowing.
Firdapse mechanism of action
Firdapse is a voltage-dependent potassium channel blocker that is known to prolong the depolarisation of the cell membrane and inhibit repolarisation, thus helping to open slow voltage-dependent calcium channels.
The action results in increased calcium transportation into the nerve endings. The higher concentration of intra-cellular calcium improves neuromuscular transmission thereby improving muscular strength.
Firdapse is available in the form of white, round 10mg tablets intended for oral administration.
Clinical trials on Firdapse
The FDA’s approval of Firdapse was based on positive results from two Phase III randomised, double-blind, placebo-controlled clinical studies, Study-1 (NCT01377922) and Study-2 (NCT02970162).
The trials enrolled a total of 64 adult patients with LEMS. The studies’ co-primary endpoints were the change in the quantitative myasthaenia gravis (QMG) score and the subject global impression (SGI) score.
In Study-1, a total of 38 patients were randomised to receive either Firdapse or placebo for more than seven days. The QMG score in patients receiving Firdapse reached a significant p-value of 0.045, while the SGI score’s p-value was 0.003.
In Study-2, 26 patients were randomised to either remain in the Firdapse treatment group or switch to placebo for four days. The QMG score in patients treated with Firdapse reached a p-value of 0.0004, while the SGI score’s p-value was 0.0003.
A difference of 6.4 points in the QMG endpoint was recorded between patients treated with Firdapse and placebo. The drug was well tolerated by patients and its safety profile was similar to previous studies.
The most common side effects reported by patients during the clinical trials were paraesthesia, diarrhoea, upper respiratory tract infection, pain in the abdomen, nausea, headache, increased liver enzymes, back pain, high blood pressure and muscle spasms.