Hycamtin is an anti-cancer drug developed by GlaxoSmithKline (GSK) as a treatment for relapsed small cell lung cancer (SCLC) in patients who have undergone and failed partial or complete first-line chemotherapy. The drug is available in injection and tablet forms.
The European Committee for Human Medicinal Products (CHMP) declared a positive opinion on the drug’s approval in November 2005. Following this, on 1 February 2006, Hycamtin received marketing authorisation from the European Commission for relapsed SCLC treatment. Hycamtin was the first European drug for the treatment of relapsed SCLC to receive authorisation.
GSK submitted the supplemental new drug application to the US Food and Drug Administration (FDA) for the use of Hycamtin in combination with cisplatin for treating advanced cervical cancer, which was accepted in February 2006. It was approved by the FDA in June 2006. In December 2006, the drug’s use in combination with cisplatin received marketing authorisation from the European Medicines Agency (EMEA).
On 15 October 2007, oral capsules of the drug were approved by the FDA on the basis of results of Phase III clinical trials. The earlier approvals were for the injection form of Hycamtin.
Small cell lung cancer
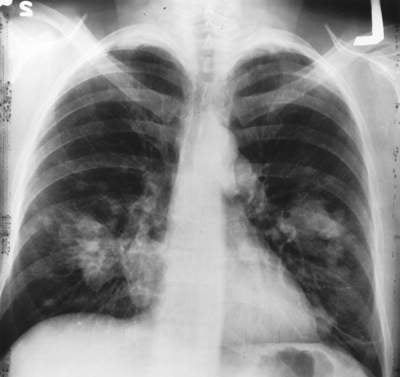
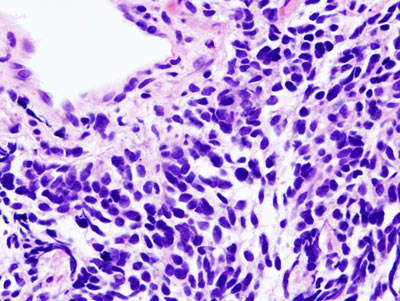
SCLC is caused by uncontrolled proliferation of cells on the surface of bronchi and this division of cells can also spread throughout the body. Surgery is not preferable in this case and chemotherapy is commonly practiced. Even after showing a positive response towards first-line therapy, the patients may again experience symptoms of the disease.
Out of 100 lung cancer cases diagnosed, 20 are SCLC cases. Out of three patients diagnosed with SCLC, one has limited disease and the remaining two have extensive disease. The survival time of patients with limited disease undergoing chemotherapy is two years. The survival time is even less at ten to 12 months in the case of patients with extensive disease undergoing chemotherapy.
SCLC is commonly caused by smoking; apart from this, environmental risk factors such as air pollution and frequent exposure to radon can cause the disease.
Targeting SCLC
Hycamtin is a topoisomerase I (topo-I) inhibitor and belongs to the topo-I inhibitor class. Topo-I is a protein produced naturally in the body, responsible for cell division in normal and cancer cells by releasing torsional strain in DNA and inducing reversible single-strand breaks.
When Hycamtin is administered, topotecan will bind to the topoisomerase I-DNA complex. It prevents the religation of single-stand breaks by permanently damaging the cell’s genetic material and preventing cell division.
Hycamtin is available as 0.25mg and 1mg oral capsules. The initial dosage of drug is prescribed as 2.3mg/m2/day for five consecutive days and is repeated after 21 days. IV Hycamtin is to be injected intravenously for five consecutive days for three weeks, whereas with oral Hycamtin patients can be treated at home.
Clinical trials show Hycamtin’s efficacy in SCLC
Phase III clinical trials were conducted under three studies. The first two studies were designed to compare the overall median survival of patients with Hycamtin to other combinations. The third study is designed to compare the overall survival and median survival between patients with Hycamtin capsules and best supportive care (BSC) and BSC alone.
The first study (protocol 090) was carried out in patients with sensitive SCLC. The trial was designed to find out the safety, efficacy and overall median survival of Hycamtin over other combination such as cyclophosphomide, doxorubicin and vincristine (CAV).
The second study was also conducted in patients with sensitive SCLC. The trial aimed to determine the safety and efficacy of Hycamtin in comparison with IV Hycamtin. The median overall survival of the two drugs was 33 and 35 weeks respectively.
The third study is a randomised, comparative, open-label and multi-centre trial in which 141 patients who showed response towards first-line chemotherapy therapy and had relapsed at least 45 days from the end of first-line chemotherapy were randomised. Some of them received BSC alone for 70 days and the rest were given Hycamtin (2.3mg/m2/day, days one through five, every 21 days) and BSC for 71 days.
The overall survival was significantly higher in those patients who received Hycamtin and BSC compared to the patients who received only BSC. The median survival was 25.9 weeks with Hycamtin plus BSC and 13.9 weeks with BSC alone. The hazard ratio was 0.6%, showing a 36% reduction in the risk of death of patients who received Hycamtin plus BSC compared to the patients who received BSC alone.
Side effects and other indications
European approval was principally based on these three key Phase III studies. During the clinical trials, the toxic effects identified with oral Hycamtin were neutropenia, thrombocytopenia, anaemia, vomiting, diarrhoea and dyspnea. Toxic deaths occurred in four patients within 30 days in the third study of Phase III clinical trials.
The FDA’s approval in June 2006 for the expanded use of the drug with cisplatin was based on trials conducted by The Gynaecologic Oncology Group (GOG). The GOG conducted a Phase III trial on women affected with stage IVB, recurrent or persistent carcinoma of the cervix. The patients included those who had recovered from the effects of chemoradiation, prior surgery or radiation.
The patients were randomly categorised into three groups, which included single-agent cisplatin (n=146, 50 mg/m2, every 21 days), Hycamtin plus cisplatin (n=147, Hycamtin 0.75mg/m2, day one to three plus cisplatin 50mg/m2 day one every 21 days), or MVAC (methotrexate, vinblastine, doxorubicin and cisplatin every 28 days). The MVAC group was closed as it was found to cause excessive toxicity.
The test results indicated a significant improvement in the overall survival.
The median survival period of patients who received Hycamtin in combination with cisplatin increased to 9.4 months from 6.5 months when receiving cisplatin alone.
Marketing commentary
Hycamtin is used in the treatment of other cancers besides SCLC. This drug is familiar to oncologists in the treatment of relapsed ovarian cancer. Hycamtin in combination with cisplatin is used in the treatment of advanced cervical cancer.
With the use of Hycamtin, SCLC patients experienced a reduction in breathing difficulties and anorexia.
The approval of Hycamtin by the CHMP will benefit patients across the UK and Europe, as well as laying the foundation to carry on future clinical research and developing programmes relating to Hycamtin.