Sanofi Pasteur’s Influenza A (H1N1) 2009 Monovalent Vaccine for 2009 Influenza A (H1N1), or swine flu, is one of the four vaccines approved by the US Food and Drug Administration (FDA) in September 2009. Other vaccines approved by the FDA are being manufactured by CSL, MedImmune and Novartis Vaccines and Diagnostics.
Sanofi filed a supplemental application for the Influenza A (H1N1) vaccine in August 2009 in response to the recommendations given by the FDA. The application was for the FDA’s assessment of the influenza A (H1N1) 2009 strain change to speed up the licensure process for the vaccine.
Sanofi received an order worth $190m in May 2009 from the US Department of Health and Human Services (HHS) to produce bulk vaccines to curb the spread of swine flu. The company received the seed virus from the Centers for Disease Control and Prevention in May 2009. Sanofi started commercial production of the vaccine in June 2009 using the virus strain to provide doses for clinical trials and to fulfil the initial order from HHS. The vaccines were distributed nationwide towards the end of October 2009.
By May 2010, Sanofi produced 87 million doses of the vaccine for the government.
2009 Influenza A (H1N1)
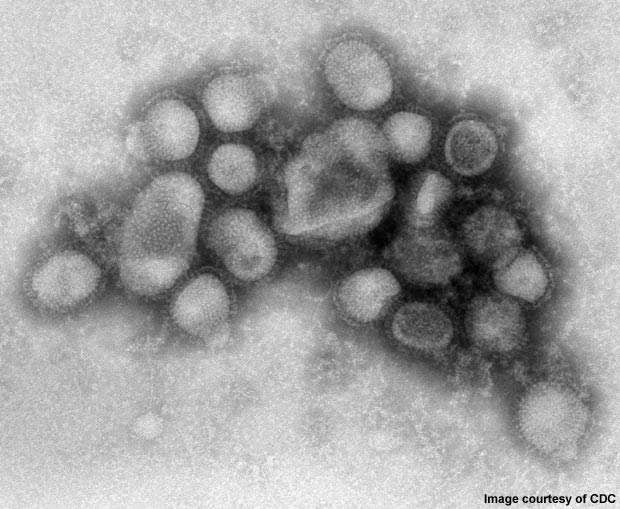
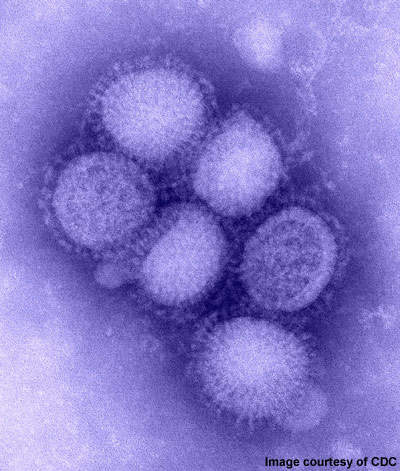
2009 Influenza A (H1N1) was first detected in Mexico in April 2009. On 11 June 2009, the WHO declared a pandemic of H1N1 flu as it had spread to several countries.
The disease was initially called swine flu since laboratory testing showed that most of the genes of the new virus were similar to the influenza viruses occurring in pigs. Further testing, however, revealed that the virus contains two genes that occur in pigs, avian genes and human genes. Scientists identified the virus as quadruple reassortant virus.
The disease is contagious and spreads from human to human and spreads faster in cold weather conditions. The age group of 15–44 years is more prone to the disease. Symptoms of the disease include fever, cough, sore throat, runny or stuffy nose, body aches, headache, chills, diarrhoea, vomiting and fatigue.
The H1N1 virus can infect the cells in the lungs resulting in severe respiratory problems. The effect of the virus can be mild, similar to those of seasonal flu, or extreme requiring hospitalisation. In severe cases, death has resulted from illness linked with the virus.
The virus has been described as very unstable and close monitoring is required to study its impact. The virus is capable of mutating and mixing with other seasonal flu strains and behaving in unpredictable ways.
Influenza A (H1N1) 2009 Monovalent Vaccine
The Influenza A (H1N1) 2009 Monovalent Vaccine contains an inactivated influenza virus. It is manufactured using the same process as Sanofi’s seasonal trivalent influenza virus vaccine or Fluzone vaccine. The vaccine is formulated to comprise 15mcg haemagglutinin (HA) of influenza A/California/07/2009 (H1N1) v-like virus.
The vaccine is licensed for individual dose presentations in syringes and vials and contains no preservative. It is also available in multi-dose vials that contain a preservative.
Clinical trials
Since Sanofi’s Influenza A (H1N1) 2009 vaccine and Fluzone vaccine are produced through the same process, initial approval of the monovalent vaccine was based on clinical studies carried out with Fluzone. The FDA also approved the influenza vaccine based on initial clinical trials which examined the monovalent vaccine’s protective effects in adults. Initial data indicated that a single 15mg dose of the vaccine generated a robust immune response in 96% of the adults aged between 18 and 64 and 56% of the adults aged 65 and above.
Full-scale clinical trials for testing the immunogenicity and safety of the Influenza A (H1N1) vaccine started in August 2009. The clinical trials will include about 2,000 subjects and will also assess the safety and potential benefits of adding an adjuvant to the vaccine.
The trials will be conducted on different sets of populations such as adults 18 and older, children in different age groups ranging from six months to 17 years and pregnant women. The trials are being funded by the National Institute of Allergy and Infectious Diseases. The clinical trials will provide additional information about the optimal dose of the vaccine in children.
In September 2009, Sanofi announced the start of randomised, double-blinded Phase II trial of the vaccine in pregnant women. The trial recruited about 120 women aged between 18 and 39, who were in their second or third trimester of pregnancy, and was carried out in six medical centres across the US. The trial was completed by May 2010.
The trial assessed the safety of the vaccine in pregnant women and the immune response after the initial dose and the second dose. It also evaluated maternal antibodies transmitted to the infants through the placenta.Results showed that the vaccine generated a strong immune response after just one dose.
As of November 2011, seven phase II trials of the vaccine have been completed in various age groups of patients.
Marketing commentary
Considering the extreme impact of the Influenza A (H1N1) virus, several governments have signed agreements for the supply of Sanofi’s monovalent vaccine. In July 2009, Sanofi obtained an order from the French Ministry of Health to supply 28 million doses of the vaccine with an option for another 28 million doses.
In September 2009, Sanofi signed an agreement for the production and supply of the vaccine with the Butantan Institute, a Brazilian biomedical research centre. The agreement includes the supply of 18 million doses of the vaccine with one million doses in final presentation and 17 million doses in bulk form. The agreement also includes an option for an additional 15 million doses of the vaccine in case the WHO directs influenza manufacturers to shift production from the Southern Hemisphere seasonal influenza vaccine to the Pandemic A (H1N1) vaccine.