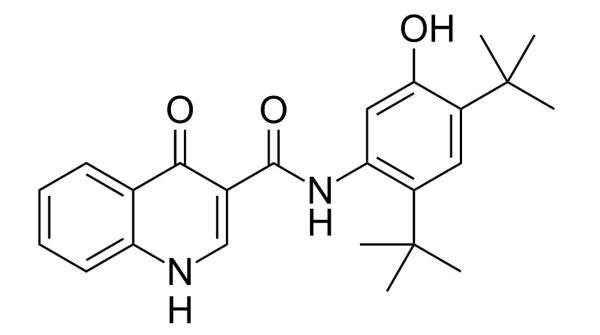
Kalydeco (Ivacaftor / VX-770) is a cystic fibrosis transmembrane conductor regulator (CFTR) potentiator indicated for the treatment of cystic fibrosis (CF). It was developed by Vertex Pharmaceuticals in collaboration with the Cystic Fibrosis Foundation.
Vertex submitted a New Drug Application (NDA) to the US Food and Drug Administration (FDA) and a marketing authorisation application (MAA) to the European Medicines Agency (EMA) in October 2011. It has requested the FDA’s priority review, which reduces the review time to six to ten months. Ivacaftor is sold under the brand name kalydeco.
In January 2012, kalydeco was approved by the FDA. It is the first FDA-approved medicine for treating underlying cause of cystic fibrosis (CF).
Cystic fibrosis (CF) genetic disease details
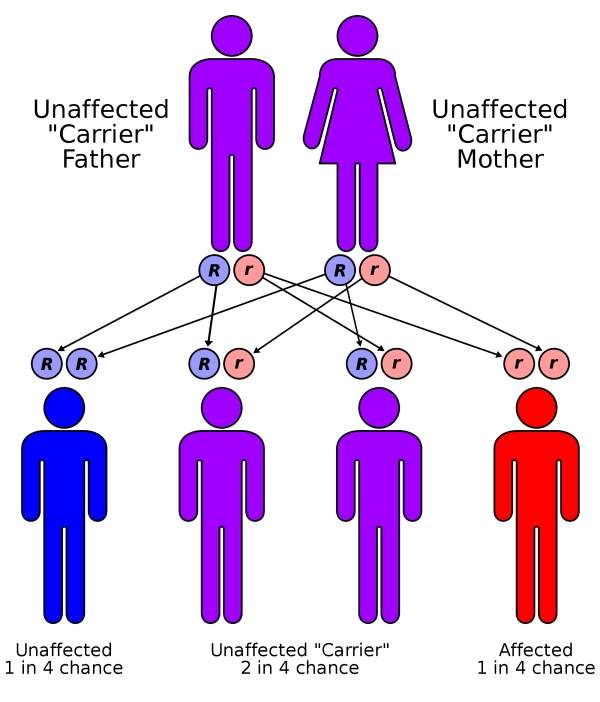
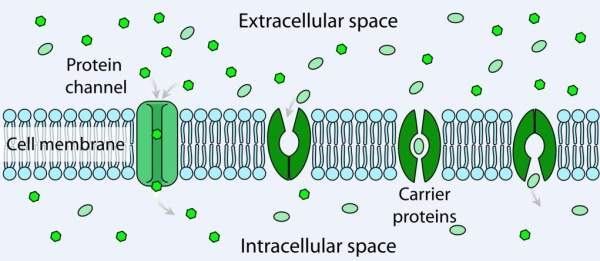
Cystic fibrosis (CF) is a genetic disease caused by a defective or missing CFTR protein leading to inadequate ion flow across cell membranes.
Most CF patients are affected by the F508del mutation in which the CFTR protein does not reach the cell surface in the required quantity. In some people, the protein is located on the surface of the cells but does not work properly. This dysfunction is known as the G551D mutation and affects about four percent of patients across the world.
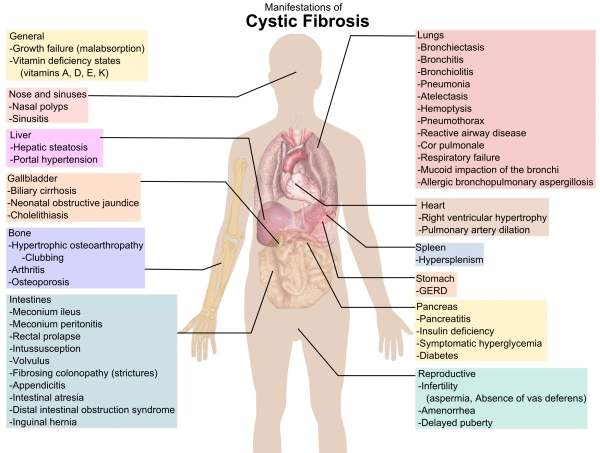
Chloride ions and sodium regulate the flow of water in the body. The absence of this causes the lungs to become dry. CF causes the secretory glands to produce abnormally thick and sticky fluid, called mucus in the lungs, pancreas, liver, digestive tract, sinuses and other parts of the body. The build-up of mucus causes repeated infections in the lungs and creates digestion problems.
Sweat glands are also affected by CF, leading to increased sweating. As a result, the body loses large amounts of salt which impacts the mineral balance in the body. This can lead to many health problems, including increased heart rates, weakness and strokes.
CF affects nearly 30,000 people in the US and 70,000 people worldwide. The main symptoms include delayed growth, weight loss, coughing or increased mucus, nausea, loss of appetite and fatigue.
Early diagnosis helps in improving the survival rate of patients. Median survival rate of a CF patient is currently more than 37 years.
Ivacaftor – cystic fibrosis transmembrane conductance regulator potentiator
Ivacaftor works by keeping the CFTR channels open to enable transportation of chloride ions across the cell membrane in CF patients. It mainly works on the G551D mutation and prevents chloride ions from exiting the cell.
Ivacaftor helps in keeping the salt balance in the body by keeping the chloride gateway open. Ivacaftor targets the root cause of CF instead of just the symptoms. If administered to newborns, ivacaftor may enable them to live a normal life.
Phase I and II clinical trials of Vertex Pharmaceuticals’ CF treatment drug
Five Phase I trials have been conducted to test the efficacy of ivacaftor in healthy subjects. One Phase I trial is currently ongoing and testing the interaction of ivacaftor and VX-809 in healthy patients. It started in October 2010 and is expected to be completed by May 2012. About 72 people were expected to be recruited for the study.
A Phase II safety trial was carried out between April 2007 and August 2008 to study the safety and efficacy of ivacaftor in CF patients with Genotype G551D.
Another Phase II trial for ivacaftor included the DISCOVER study. It enrolled 140 patients who were 12 years or older who had two copies of the F508del-CFTR mutation. The study started in August 2009 and was completed in July 2010.
NDA and MAA submission was based on the DISCOVER and two Phase III trials – ENVISION and STRIVE.
ENVISION was carried out in 52 children aged between six and 11. Results from the study indicated that kalydeco was effective in improving lung function in patients. The mean absolute improvement in lung function in patients was 15.1% from baseline compared to placebo. The study also indicated increases in levels of sweat chloride levels.
The STRIVE study recruited 161 adolescents and adults aged 12 years or more suffering with CF. After 48 weeks of treatment with ivacaftor, the mean absolute improvement in lung function was 10.6% from baseline, compared to placebo.
Another Phase III trial, PERSIST, is being carried out in patients who have completed the ENVISION and STRIVE studies. About 200 patients have been enrolled for the study which started in July 2010. The study will test the long-term efficacy of ivacaftor and is expected to be completed by May 2013.
In June 2011, Vertex announced results from part one of an ongoing Phase II study aimed at evaluating the safety and tolerability of ivacaftor in combination with the CFTR corrector – VX-809. The study started in October 2010 and enrolled 62 CF patients aged 18 years or older. Results from part one of the study indicated a two-fold decrease in sweat chloride.
Part two of the Phase II trial will enrol 100 patients who will be administered only VX-809 for four weeks followed by VX-809 in combination with ivacaftor for four weeks. Patient enrolment began in October 2011. The study is expected to be completed by July 2012.
Two more Phase II trials are currently ongoing. One study is recruiting patients 12 years or older and will test the effect of ivacaftor on CF patients with G551D mutation. The study started in October 2010 and is expected to be completed by February 2013.
The second Phase II trial started in January 2011 and enrolled 24 patients. It measured the lung clearance index in CF patients aged six years or older. The study was completed in December 2011.
Marketing commentary for Vertex Pharmaceuticals’ kalydeco (ivacaftor)
Current treatments available for CF include inhaled spray which contains copies of the CF gene. Another treatment includes protein repair therapy which helps in repairing the defective CFTR protein.
Ivacaftor is the first treatment to target the basic cause of CF and enable patients to live a longer and healthy life.