Mayzent (siponimod) is one of the first drugs to be approved for the treatment of relapsing forms of multiple sclerosis (MS).
The drug was developed by Swiss multinational pharmaceutical company Novartis.
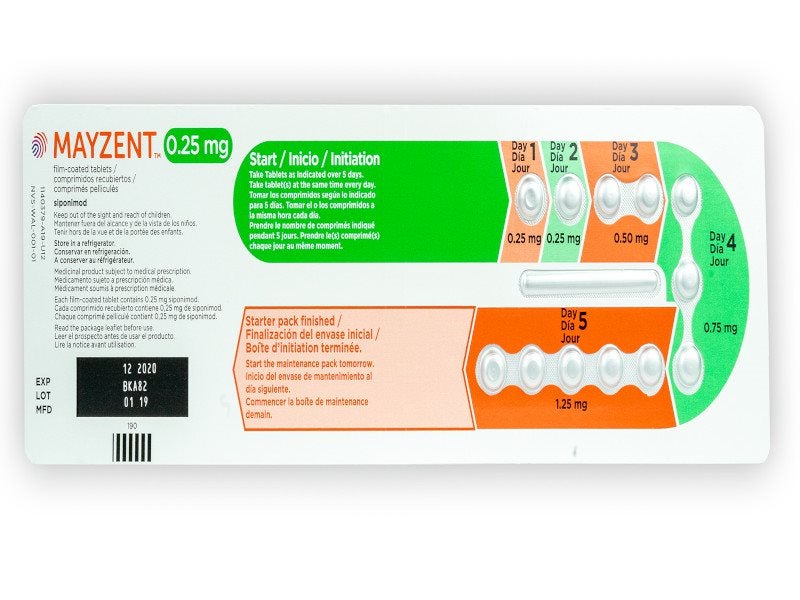
It is available as round biconvex film-coated tablets in three distinct doses of 0.25mg, 1mg, and 2mg. The 0.25mg tablet is pale red in colour while the 1mg tablet is violet white and the 2mg tablet is pale yellow.
Regulatory approvals for Mayzent
The US Food and Drug Administration (FDA) and European Medicines Agency (EMA) accepted new drug application and marketing authorisation application submissions for the drug in October 2018. The FDA approved the drug in March 2019.
Novartis received a positive opinion from the EMA’s Committee for Medicinal Products for Human Use for Mayzent in November 2019.
The European Commission authorised Mayzent for the treatment of adult patients with SPMS with active disease shown by relapses or imaging indications of inflammatory activity in January 2020.
Mayzent was approved by Health Canada in March 2020 and by the Japanese Ministry of Health, Labour, and Welfare in June 2020.
MS causes and symptoms
MS is a chronic autoimmune disease that affects the brain and spinal cord. It is caused due to demyelination of the insulating myelin sheath around the nerve cells in the brain and spinal cord.
Demyelination disables the central nervous system’s (CNS) ability to communicate, leading to muscular weakness, visual problems, motor skills dysfunction, and cognitive dysfunction.
MS is categorised into relapsing-remitting MS (RRMS), primary progressive MS, and SPMS.
RRMS is the initial form of MS, which progresses into SPMS. An estimated 85% of all the diagnosed cases of MS are classed as SPMS, which is characterised by a gradual worsening of the nervous system function over time.
MS is estimated to affect approximately 2.3 million people globally.
Mayzent’s mechanism of action
Mayzent is a sphingosine 1-phosphate (S1P) receptor modulator that reduces the migration of lymphocytes in the CNS.
The drug is known to bind with high affinity to S1P receptors one and five, which are present in CNS cells. It blocks the lymphocytes’ capacity to move out from lymph nodes, reducing the lymphocyte count in the peripheral blood and forcing them to move into the CNS.
Clinical trials on Mayzent
The FDA’s approval of Mayzent was based on a Phase III, randomised, placebo-controlled clinical study named EXPAND. A total of 1,652 patients with SPMS were enrolled in the study from 31 countries to evaluate the safety and efficacy of the drug.
Patients were randomised in a 2:1 ratio to receive either siponimod or placebo. The primary endpoint of the study was three-month confirmed disability progression (CDP) events.
Results from the study showed that Mayzent significantly reduced the three-month CDP risk in patients by 21%. The drug also showed a 33% reduction in the three-month CDP risk compared to placebo in patients that had relapsed activity two years before screening for the trial.
In addition, the drug significantly delayed the six-month CDP risk by 26% in patients receiving Mayzent, compared to placebo. The patients on Mayzent also demonstrated a reduced annualised relapse rate (ARR) of 55%. Other improvements were also seen in several disease symptoms such as cognitive skills and brain shrinkage.
Some of the common adverse events reported in the patients during the clinical trial were headache, high blood pressure and increase in transaminase.
The long-term effectiveness and safety of Mayzent in patients with SPMS were compared with a placebo in the EXPAND open-label extension trial. Data from the five-year study showed that treatment with Mayzent helped in reducing ARR by 52% compared to placebo.
Mayzent also significantly reduced demyelination and supported preclinical findings on remyelination.
Marketing commentary on Novartis
Novartis is a pharmaceutical company based in Switzerland. The key therapeutic areas of focus for the company include cardiometabolic, hepatology, musculoskeletal diseases, ophthalmology, neuroscience, nephrology, respiratory, oncology, immunology, and dermatology.
The global operating divisions of the company include Alcon and Sandoz, which is one of the world’s largest generic pharmaceutical companies.