Lumoxiti (Moxetumomab pasudotox) is an investigational anti-CD22 recombinant immunotoxin developed as a potential treatment for relapsed/refractory hairy cell leukaemia (HCL) patients previously treated with at least two lines of therapy.
The drug was originally discovered by the National Cancer Institute, and later developed by US-based pharmaceutical company Medimmune.
Medimmune submitted a biologics license application (BLA) to the US Food and Drug Administration (FDA) in April 2018. The FDA approved the drug under its priority review programme in September 2018.
Lumoxiti (Moxetumomab pasudotox) has also received orphan drug designation from the European Medicines Agency (EMA) for the treatment of HCL.
Hairy cell leukaemia causes and symptoms
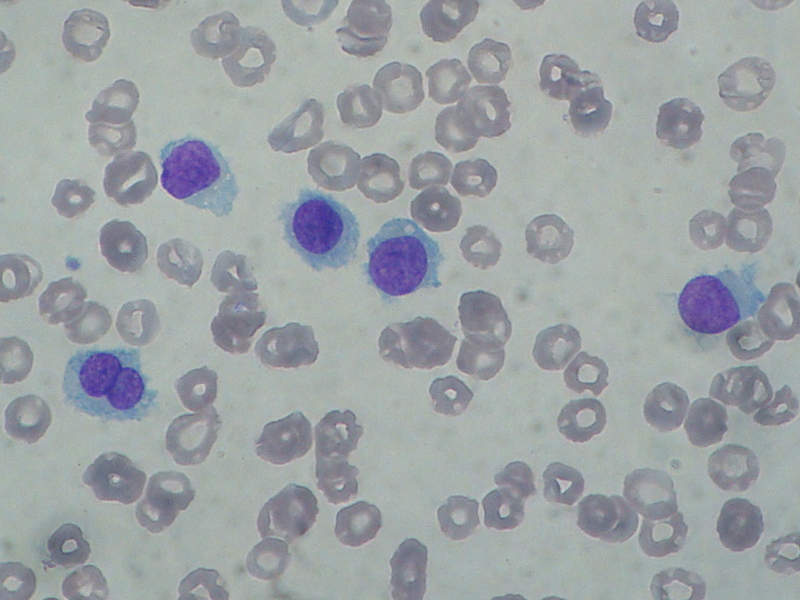
HCL is a rare chronic blood cancer that causes bone marrow to generate excessive abnormal B-cells, preventing the production of healthy blood cells.
The abnormal B-cells of HCL patients look hairy under a microscope. The cause of the disease is unknown, but research suggests that exposure to radiation and certain agricultural and industrial chemicals increases the chances of HCL.
Some of the common symptoms of the disease are bruising and bleeding, recurring infection, weight loss, fatigue, weakness and an uncomfortable feeling in the abdomen area.
Around 1,000 people a year in the US are diagnosed with HCL. The relapse rate of the disease is high with very few treatment options available.
Lumoxiti (Moxetumomab pasudotox) mechanism of action
Lumoxiti (Moxetumomab pasudotox) is an investigational anti-CD22 recombinant immunotoxin. It comprises a binding portion of anti-CD22 antibody fused to a toxin called PE38, which is a 38kDa fragment of Pseudomonas exotoxin A.
The drug functions as a CD22 antigen inhibitor that binds to CD22 and releases the toxin into the cell. The toxin inhibits the protein translation process of the cancer cells, leading to cell death.
Recombinant immunotoxin is administered intravenously to the cancer patients so that the drug can reach every cell.
Clinical trials on Lumoxiti
The FDA’s approval of Lumoxiti was based on data from the Phase III clinical study known as ‘1053’. The single-arm, open-label, multi-centre study enrolled 80 patients with relapsed or refractory HCL and was carried out across 34 locations in 14 countries. The primary endpoint of the trial was durable complete response.
The trial met the primary endpoint with a 30% durable CR rate observed in patients receiving Lumoxiti (Moxetumomab pasudotox). The drug also achieved 75% objective response rate and a 41% CR rate.
A total of 73% of the patients achieved a durable response, while 82% achieved a negative minimal residual disease (MRD) rate. Hematologic remission was also observed in 80% of patients.
The patients achieved hematologic remission at a median time of 1.1 months.
Nausea, headaches, peripheral oedema, and pyrexia were the common treatment-related adverse events noticed during the clinical trial.
Marketing commentary on Medimmune
Headquartered in Gaithersburg, Maryland, Medimmune is a wholly-owned subsidiary of AstraZeneca.
The company focusses on core therapeutic areas, oncology, inflammation and autoimmune and cardiovascular disorders. It has more than 120 biologics in research and development (R&D) for various indications, including cancers, asthma, lupus and chronic obstructive pulmonary disease (COPD).