Nulojix (Belatacept) was developed by Bristol Myers Squibb and is indicated to prevent the rejection of the organ in patients who have undergone kidney transplantation. The powdered form of the drug is produced for use in the preparation of solution for intravenous injection.
The drug was approved by the US FDA and in the European Union in June 2011 for use in adult patients undergoing kidney transplantation. The approval was based on two phase III studies, Benefit and Benefit-Ext.
Acute organ rejection in transplant patients
Kidney transplantation is usually performed in patients with chronic kidney diseases such as polycystic kidney disease.
After a major transplantation, the immune system considers the new organ as a foreign body and hence acts against it as a defence mechanism.
Most of the patients undergoing transplantation face organ rejection. The organ rejection may be either acute rejection or a chronic rejection.
Acute rejection occurs in most of the patients undergoing organ transplantation.
The new organ, unable to thwart the action, starts showing symptoms of organ failure. Acute rejection usually occurs within weeks or months of transplant.
The symptoms of organ rejection after a kidney transplant include decrease in urine output, fever, increase in weight, increase in blood pressure and pain near the kidney area.
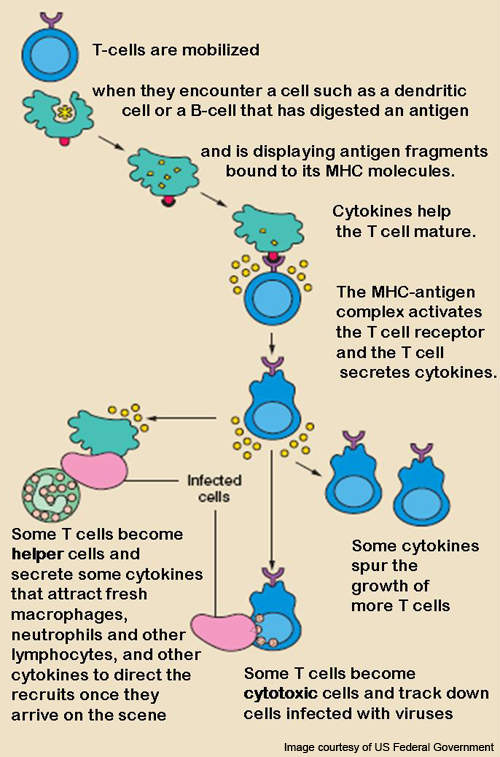
Belatacept – mechanism of action
T lymphocytes (T-cells), a type of white blood cell, are usually activated after a week of transplant.
T-cells react with antigens to get activated and form an immune response or alloreaction.
CD28 is the protein required for the activation of T-cells.
The alloreaction occurs after differentiation of T-cells and these alloreactive T-cells cause the breakdown of the transplanted organ or may even cause necrosis of the new organ.
Belatacept, the substance present in Nulojix, has the ability to block the T-cell selectively. It binds to the proteins CD80 and CD86 of the antigen presenting cells and hinders the CD28, thereby preventing the activation of T-lymphocytes.
Side affects caused by the drug are Anemia, constipation, kidney infection, swelling of ankles, legs and feet.
Clinical trials of nulojix / belatacept
Phase I clinical trials on nulojix were initiated in December 2007 and enrolled 47 healthy people. The trial evaluated the immunogenicity, safety, efficacy and pharmacokinetics of the drug in healthy people. It was completed in August 2008.
Phase II trials to evaluate the safety and efficacy of the drug were initiated in March 2001 and enrolled 230 patients.
Patients were administered either belatacept in combination with cellcept, simulect and corticosteroids or were given cyclosporine. The study was completed in January 2004.
Another Phase II trial was conducted in 173 kidney transplant patients already being administered with cyclosporine. The aim of the trial was to observe if the organ rejection could be prevented by administering belatacept instead of cyclosporine. The trial was initiated in January 2007 and expected to be completed by May 2011.
A Phase II trial was also conducted to observe the pharmacokinetics and safety of the drug in patients being administered with belatacept based medications.
The trial which was initiated in March 2008 is expected to be completed by August 2011.
Phase III trials and Benefit / Benefit-Ext studies
Two phase III studies, namely Benefit and Benefit-Ext, were conducted in kidney transplant patients for three years.
Both the studies were conducted to evaluate the safety and efficacy of the drug, and to compare the effect of belatacept to that of the cyclosporine.
The Benefit-Ext study enrolled 540 patients, and was initiated in March 2005 and reached primary completion in May 2008. It was conducted in patients who received kidneys from diseased patients. The Benefit study enrolled around 660 patients and was initiated in June 2006.
The study was conducted in patients who received kidneys from living donors. The primary data of the trial was released in June 2008. Both the studies showed that belatacept was superior to cyclosporine in terms of graft survival.
Another trial was initiated in July 2010 in type 1 diabetic patients who have undergone simultaneous islet and kidney transplantation. The trial will evaluate the effect of the drug on kidney functioning, the survival rate, and the safety and efficacy of the drug. The trial is expected to be completed July 2012.
A long term study called Enlist (evaluating nulojix long-term safety in transplant) will be conducted to evaluate the safety and efficacy of the drug in belatacept administered patients.
The trial will enrol patients undergoing belatacept treatment and will evaluate the rate of occurrence of post-transplant lymphoproliferative disorder (PTLD) and PML (an infection leading to death in the patients).
Market commentary
Belatacept is the first drug to be approved for the prevention of organ rejection in the last ten years. It is expected to generate annual sales of $350m to $500m by the end of 2015.