Pifeltro (doravirine) is indicated for the treatment of adult patients with Human Immunodeficiency Virus-1 (HIV-1) infection.
Developed by US-based pharmaceutical company Merck, Pifeltro™ is administered in combination with other antiretroviral medicines for treatment-naïve adult patients.
Merck submitted a new drug application (NDA) for Pifeltro to the US Food and Drug Administration (FDA) in October 2017, which was accepted for review in January 2018. The drug was approved in August 2018, ahead of the set target action date of October 2018.
Pifeltro is currently being reviewed by the European Medicines Agency (EMA).
HIV-1 disease causes and symptoms
HIV infection is caused by a virus that destroys CD4 T-cells, which play a crucial role in fighting disease. This weakens the immune system and makes the patient vulnerable to infections and diseases.
Out of the two types of HIV infection, HIV-1 is the most widespread globally and more virulent and infective than HIV-2.
HIV infection can spread through infected blood, semen or vaginal secretions. Common signs and symptoms include fever, headache, muscle and joint pain, sore throat, swollen lymph glands and rashes.
An estimated 36.9 million people were living with HIV infection in 2017, while another 1.8 million are newly infected with the disease.
Pifeltro’s mechanism of action
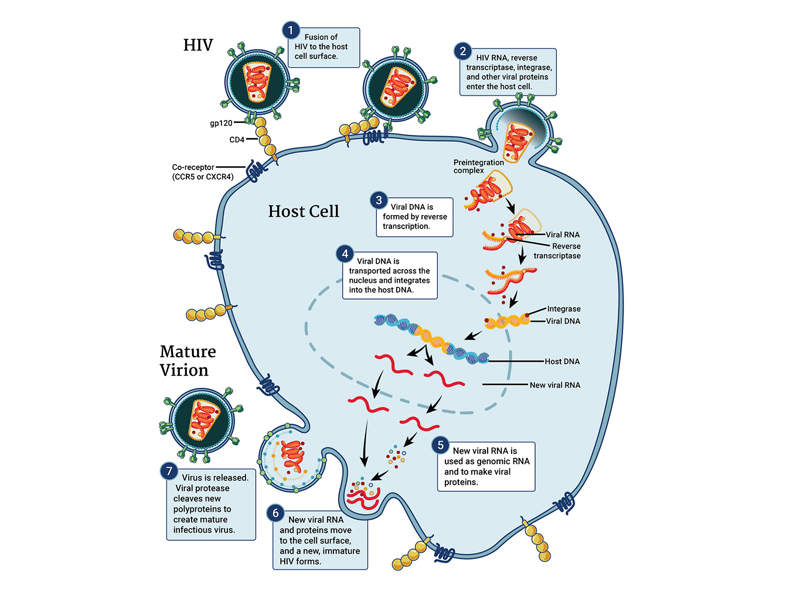
Pifeltro™ is an antiretroviral drug containing doravirine as the active ingredient. Doravirine is a pyridinone non-nucleoside reverse transcriptase inhibitor of HIV-1 virus.
Pifeltro™ inhibits the HIV-1 reverse transcriptase enzyme in the virus, which results in the inhibition of HIV-1 replication. HIV-1 reverse transcriptase enzyme plays a crucial role in the replication of the virus in the body. The drug does not block other enzymes such as human cellular DNA polymerases α, β, and mitochondrial DNA polymeraseγ.
Pifeltro™ is available in the form of 100mg film-coated tablets for oral administration.
Clinical trials on Pifeltro
The FDA’s approval of Pifeltro was based on the positive results of two 48-week Phase III pivotal clinical trials named DRIVE-FORWARD and DRIVE-AHEAD.
The DRIVE-FORWARD trial evaluated the safety and efficacy of Pifeltro™ in 766 previously untreated patients with HIV-1 infection. The primary endpoint of the randomised, multi-centre, double-blind, active-controlled clinical trial was non-inferior efficacy. Patients were randomised to receive either Pifeltro™ or darunavir (800mg) plus ritonavir (100mg) once daily, each in combination with emtricitabine or abacavir.
At week 48, approximately 84% of people treated with Pifeltro™ achieved viral suppression compared with 80% of eople treated with darunavir plus ritonavir. Out of the 20% of patients in the trial with a high viral load, 77% of Pifeltro-treated patients achieved viral suppression at week 48, compared with 74% of patients treated with darunavir plus ritonavir.
The rate of discontinuation of the treatment due to adverse events during the trial was low. The common adverse events observed in patients treated with Pifeltro were nausea, headache, fatigue, diarrhea, dizziness, abdominal pain and abnormal dreams.
A total of 728 patients were enrolled in DRIVE-AHEAD trial and radomised to receive either a combination of doravirine along with lamivudine and tenofovir disoproxil fumarate (3TC/TDF) or a combination of efavirenz with efavirenz/emtricitabine/tenofovir disoproxil (FTC/TDF) once-daily.
At week 48, 84% of people treated with doravirine and 3TC/TDF achieved viral suppression, compared with 81% of people receiving efavirenz with FTC/TDF.
Marketing commentary on Merck
Merck is a US-based pharmaceutical company headquartered in New Jersey. The company operates in more than 140 countries and is known as MSD outside the US and Canada.
The main focus areas of the company include diabetes, oncology, cardiovascular disease, infectious diseases, respiratory disease, immunology and animal health products. Merck offers more than 50 prescription products in its portfolio.