Polivy™ (polatuzumab vedotin-piiq) is an antibody-drug conjugate indicated for the third-line treatment of adults with relapsed or refractory (r/r) diffuse large B-cell lymphoma (DLBCL).
Developed by Genentech the drug has been devised using the Seattle Genetics’ antibody-drug conjugate technology. It is administered in combination with bendamustine plus Rituxan® (rituximab).
Polivy™ received priority medicines (PRIME) designation from the European Medicines Agency (EMA) to treat r/r DLBCL in June 2017. The medicine also holds orphan drug and breakthrough therapy designations from the US Food and Drug Administration (FDA).
The FDA granted priority review status to Genentech’s biologics license application (BLA) for Polivy™ in February 2019. The drug received accelerated approval from the FDA in June 2019.
Polivy™ is a white to greyish-white lyophilised powder available in 140mg strength in a single-dose vial for reconstitution and further dilution for intravenous use.
Diffuse large B-cell lymphoma causes and symptoms
DLBCL is one of the most common and aggressive types of non-Hodgkin’s lymphoma (NHL). It originates in the white blood cells (lymphocytes) and spreads via the lymph nodes.
The disease can occur in childhood but is most commonly diagnosed in patients aged 60 years or more. DLBCL accounts for one in three cases of NHL and is newly diagnosed in 123,000 individuals a year, worldwide.
Some common symptoms of the disease are a lump in the armpit, groin or neck; unexpected weight loss, fever, chest pain, dyspnoea and itching.
Polivy’s mechanism of action
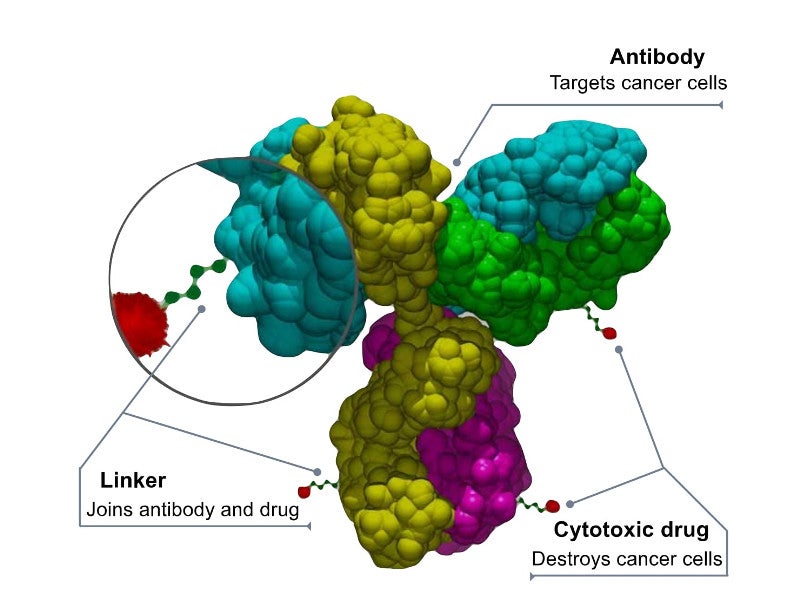
Polivy™ is a first-in-class antibody-drug conjugate targeting CD79b, a protein expressed in most of the B-cells. The antibody-drug conjugate combines a monoclonal antibody (mAb) and a potent cytotoxic agent with a linker system.
The mAb of Polivy™ binds to the CD79b protein and destroys the B-cells by delivering a cytotoxic agent into them.
Clinical studies on Polivy
The FDA’s approval of Polivy was based on the positive outcomes of the second phase of an ongoing global, phase Ib/II, multi-centre, open-label clinical study named GO29365 in patients with r/r B-cell lymphomas.
The clinical study is being performed to evaluate the safety, efficacy, and tolerability profile of Polivy™ when used in combination with bendamustine and Rituxan® (BR) or Gazyva® (obinutuzumab).
A total of 80 patients were randomised in the clinical study to receive either Polivy™ with BR or BR alone for a period of six 21-day cycles. The primary endpoint of the study is the complete response rate at the end of the treatment.
The results from the study demonstrated that 40% of patients treated with Polivy™ plus BR achieved a complete response (CR), compared to 18% of the patients treated with BR alone.
Approximately 45% of patients that were treated with Polivy™ plus BR achieved objective response at the end of treatment, compared to only 18% of patients treated with BR alone.
The response lasted for at least six months in 64% of the Polivy™ plus BR-treated patients that achieved either CR or partial response, compared to 30% of those treated with BR alone.
The patients treated with Polivy™ plus BR achieved an overall survival of 12.4 months compared to 4.7 months in patients treated with BR alone.
The most common adverse events (AE) reported in patients during the clinical trials were anaemia, neutropenia, thrombocytopenia, peripheral neuropathy, fatigue, diarrhoea, increased body temperature, decreased appetite and pneumonia.