Afinitor (everolimus), formerly known as RAD001, is a macrolide antibiotic derived from rapamycin that is being investigated by Novartis as a potential treatment for various solid tumours, including advanced kidney cancer. Afinitor is also used to treat pancreatic neuroendocrine tumours, gastric cancer and breast cancer.
Marketed under the brand name Certican, Novartis’ everolimus is already an approved medication for the prevention of organ rejection in patients undergoing heart and kidney transplantation.
Besides potent immunosuppressant effects, everolimus also inhibits mTOR protein, and it is this property that is being exploited in the development of Afinitor (everolimus) as a potential anti-cancer medication.
Phase III clinical trials on Afinitor were aborted by the data monitoring committee as interim results proved 67% progression-free survival in patients who received the drug, compared to placebo, and thus the trials met the principal endpoint.
The US Food and Drug Administration (FDA) and European Commission (EC) granted regulatory approval for the use of Afinitor in treating renal cell cancer in March and August 2009 respectively. The EC’s decision is applicable in 27 EU states. Afinitor was also approved in Switzerland in 2009.
In January 2010, Novartis announced that Afinitor was approved in Japan for treating people affected with non-resectable, metastatic renal cell cancer.
The drug was approved by the FDA in October 2010 for the treatment of subependymal giant cell astrocytoma, a benign brain tumour associated with tuberous sclerosis that affects children as well as adults. In May 2011, the drug was approved by the Swiss Agency for Therapeutic Products (Swissmedic) to treat subependymal giant cell astrocytoma in patients aged three and older. It will be available under the trade name Votubia.
In April 2011, Novartis also filed a new drug application for Afinitor for the treatment of advanced neuroendocrine tumours of pancreatic gastrointestinal, lung or pancreatic origin. The FDA approved the drug in May 2011, making it the first in the US to be cleared for this indication.
Afinitor obtained approval from the EC for the same indication in September 2011. The approval is applicable in all 27 countries of the European Union, as well as Iceland and Norway. Novartis has filed a number of other regulatory applications for Afinitor, which are currently under review with health authorities across the world.
Targeting mTOR
Mammalian target of rapamycin, or mTOR, is a protein kinase that regulates cell growth, cell proliferation, cell motility and cell survival, as well as protein synthesis.
Studies suggest that the mTOR growth pathway may be hyperactive in some cancers, making it an attractive cancer therapy target. Novartis’ Afinitor is a serine-threonine kinase inhibitor of mTOR, which prevents tumour cell division and blood vessel cell growth.
Prior to Afinitor’s approval, Wyeth’s mTOR inhibitor temsirolimus (Torisel ҩ was the only marketed cancer therapy that specifically inhibited mTOR. Torisel Ҡwas approved for the treatment of advanced renal cell cancer in 2007.
Afinitor shows efficacy in renal cell cancer
The efficacy and safety of Afinitor was demonstrated in the Phase III RECORD-1 (REnal Cell cancer treatment with Oral RAD001 given Daily) trial. The trial was multi-centre randomised, double-blind and placebo-controlled, and enrolled more than 400 patients with advanced renal cell cancer whose disease had progressed despite prior treatment with a range of first-line therapies. This included treatment with two of the newest drugs for renal cell cancer, sorafenib and sunitinib.
Interim results from RECORD-1 showed that treatment with Afinitor more than doubled the time to tumour progression after failure of first-line therapy. An independent data monitoring committee stopped the RECORD-1 trial in early 2008 as Afinitor was the first drug to show significant benefits after the failure of approved therapies sorafenib and sunitinib. Median progression-free survival (the primary endpoint) was four months for patients in the Afinitor arm, compared with 1.9 months in the placebo arm (p<0.0001), a 70% reduction in the risk of cancer progression.
The study findings were presented at the 44th Annual Meeting of the American Society of Clinical Oncology (ASCO) in Chicago, US, in May 2008. The updated RECORD-1 data was presented in September 2008 at the 33rd European Society for Medical Oncology Congress in Stockholm, Sweden.
No significant differences were observed with respect to secondary efficacy endpoints, which included overall survival and objective response rate. A central review of the patients evaluable for best percentage change in target lesions showed evidence of tumour shrinkage in 50% of Afinitor-treated patients during the double-blind phase, compared with 8% of placebo recipients.
Afinitor appeared generally well tolerated by patients in this study, with 6% prematurely discontinuing because of adverse effects.
Potential in other cancers
In addition to renal cell cancer, Afinitor is also being investigated for its potential to treat other cancers.
Studies have been conducted to see whether it can bring therapeutic benefit to patients with pancreatic endocrine tumours. In June 2009, results from a Phase II trial involving 160 patients with pancreatic neuroendocrine tumours showed that Afinitor may help in stabilising tumour growth. The study, named RADIANT-1 (RAD001 In Advanced Neuroendocrine Tumours), was conducted with Afinitor in combination with Sandostatin and with Afinitor as monotherapy.
RADIANT-2, a Phase III trial, was conducted to observe the safety and efficacy of the drug in combination with octreotide LAR compared to a placebo. A total of 429 patients suffering from advanced neuroendocrine tumours were enrolled. Patients were administered either Afinitor in combination with octreotide LAR or a placebo with octreotide LAR. The primary endpoint of progression-free survival was not met.
RADIANT-3, another Phase III trial, was carried out to further investigate Afinitor as a treatment option for patients affected with pancreatic neuroendocrine tumours. Patients were given either 10mg of the drug or a placebo orally. The trial showed Afinitor increased the average survival time without cancer progression from 4.6 to 11 months
Other cancers for which Afinitor is being considered include carcinomas of the stomach and lung.
Clinical trials
Afinitor underwent clinical trials in three phases, which included trials to test the drug’s efficacy in different cancer types.
A multi-centre Phase I clinical trial of daily and weekly everolimus in combination with weekly paclitaxel and trastuzumab was carried out in patients with HER2 over-expressing metastatic breast cancer with prior resistance to trastuzumab.
A randomised, double-blind and placebo-controlled Phase II trial was conducted after enrolling 270 postmenopausal women as a part of Afinitor’s breast cancer clinical trials. The clinical response rate with Afinitor (68%) when combined with letrozole (Femara) was higher than that of letrozole alone (59%). Ultrasound results proved in favour of Afinitor.
As a part of Afinitor’s clinical trials in evaluating the drug’s efficacy in gastric cancer patients, a multi-centre Phase II trial involving 54 patients was conducted in Japan. Tumour growth was halted in 55% of advanced gastric cancer patients. Severe adverse events included anaemia, hyponatraemia, raised liver function, fatigue, stomatitis, anorexia, hyperglycaemia, hypophosphataemia, ileus and lymphopenia.
Phase I and Phase II results were also presented at the 44th annual meeting of the ASCO in Chicago, US, in May 2008. Phase II trial results released in January 2009 revealed efficacy in patients with advanced gastric cancer. Phase II study findings also revealed tumour shrinkage when the drug was given in combination with letrozole to post-menopausal women diagnosed with oestrogen receptor-positive breast cancer.
Results from two Phase I-II trials showed efficacy when Afinitor was combined with trastuzumab and other chemotherapy agents. This combination overcame resistance to trastuzumab and achieved complete responses in a few patients and partial responses in majority of patients.
Results of a randomised Phase II study conducted on patients suffering from advanced metastatic breast cancer were released in December 2010. Patients were administered with either everolimus in combination with tamoxifen or tamoxifen alone. Everolimus with tamoxifen had an increased median time to disease progression of 8.6 months when compared to 4.5 months median time demonstrated by patients given tamoxifen alone. The study met its primary endpoint.
Two Phase III trials, BOLERO-1 and BOLERO-2, have been initiated to further study the drug for the treatment of metastatic breast cancer.
BOLERO-1 is a double-blind and placebo-controlled multicentre study to evaluate everolimus in combination with trastuzumab and paclitaxel in 717 women with HER2-positive locally advanced or metastatic breast cancer. The trial started in September 2009 and is expected to be completed by October 2012.
The second Phase III trial, BOLERO-2, recruited 724 postmenopausal women with ER+HER2- advanced breast cancer who recurred or who were earlier treated with letrozole or anastrozole. It was conducted across 189 sites in 24 countries worldwide. The study evaluated everolimus in combination with exemestane against placebo plus exemestane.
In July 2011, Novartis announced that BOLERO-2 had met its primary endpoint and significantly extended the progression-free survival rate in patients to 10.6 months.
A Phase II trial was conducted on children and adults suffering from tuberous sclerosis to demonstrate that Afinitor reduced the size of subependymal giant cell astrocytomas. A total of 28 patients were enrolled and administered with an oral dose of 3mg of everolimus a day. The study was conducted for a period of 21.5 months. The study met its primary endpoint of change in the number of subependymal giant cell astrocytoma lesions.
Two other Phase III trials, EXIST-1 and EXIST-2, have also been completed. EXIST-1 observed the effect of the drug on tumour shrinkage, seizures and skin abnormalities associated with TS. In July 2011, the study met its primary endpoint and showed that patients treated with everolimus experienced 50% or greater reduction in tumours. About 117 patients were recruited for the study across ten countries.
EXIST-2 was initiated to observe the effect of the drug in 118 patients with angiomyolipoma. The trial was conducted in 11 countries: Australia, Belgium, Canada, France, Germany, Italy, the Netherlands, Poland, Russia, the UK and the US. It met its primary endpoint in September 2011, demonstrating tumour reduction in 42% of the patients compared with zero in the placebo group.
Marketing commentary
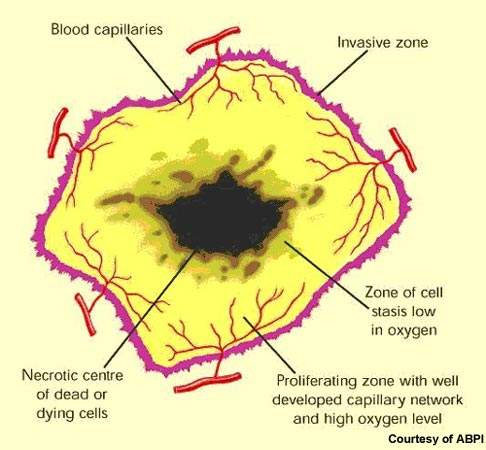
Renal cell cancer, which develops in the renal tubules, is the most common cancer of the kidney. It accounts for about 2–3% of all new cancers. Surgery to remove the tumour is standard treatment, and can be effective if the tumour is confined to the kidney.
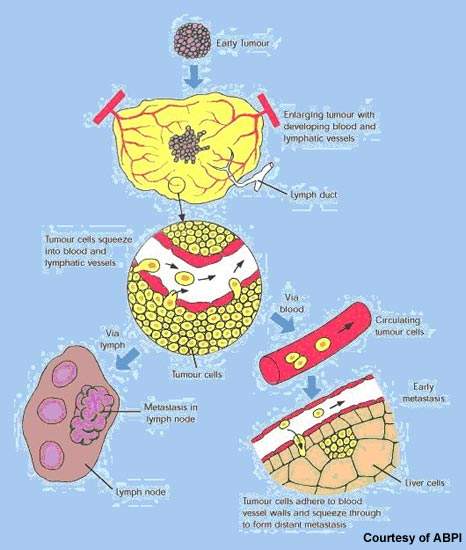
However, if the tumour recurs following surgery prognosis is often poor. Generally resistant to radiotherapy and chemotherapy, renal cell cancer has proved one of the more difficult cancers to treat once the disease has spread.
Some important advances have been seen in the treatment of renal cell cancer with the arrival of targeted therapies such as the multi-kinase inhibitors sorafenib and sunitinib. Although not curative, they can significantly prolong progression free survival in advanced renal cell cancer. As results from the RECORD-1 trial demonstrate, treatment with Afinitor may further extend progression-free survival in patients with metastatic renal cell cancer after failure with these newer drugs.