Even though Humira’s US and Japan patents have expired in 2017, and most of its EU patents soon to expire in 2018, AbbVie has a wide assortment of intellectual property and patents that will protect its blockbuster from competitive market entry for the next few years.
AbbVie’s chief executive officer (CEO) Richard Gonzalez mentioned to analysts in the Q3 earnings conference that the compabny plans to protect its blockbuster from biosimilar competitors. The firm has more than 70 patents covering the manufacturing, formulation, and dosage of Humira up until 2022. In addition, Humira has also held Orphan Drug designation for three different indications, which will extend its US market exclusivity until 2021.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
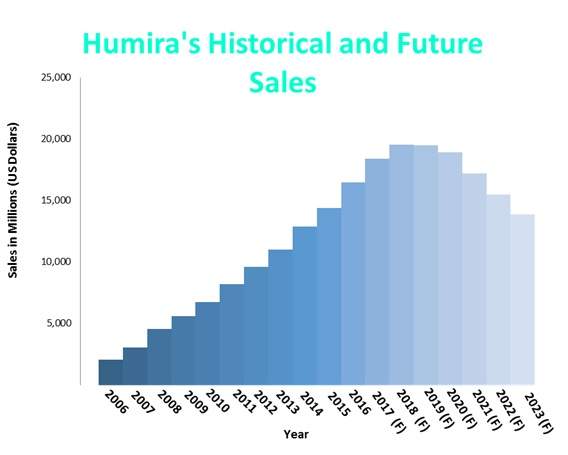
In addition, brokers predict sales of Humira will remain above $16 billion until 2021 and will not be affected by biosimilar competitors at least until 2021. However, with AbbVie facing many legal shots at the blockbuster from its competitors, it has recently lost its bi-weekly 40mg injection technique patent to Coherus Biosciences.

Figure 1: AbbVie’s Humira sales forecasted to rise for three years despite its patents expiring since 2016.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData




