Rexulti (brexpiprazole) is a newly-discovered psychotropic compound indicated as an add-on therapy for major depressive disorder (MDD) and as a treatment for schizophrenia. The drug was discovered by Otsuka and is being co-developed with Lundbeck.
Rexulti was approved by US Food and Drug Administration (FDA) in July 2015 as an adjunctive treatment for adults with MDD and as a treatment for adults with schizophrenia. The new drug application (NDA) of Rexulti was accepted by the FDA in September 2014.
The drug will be available in the US in early August 2015 and will be co-marketed by the two companies. It will be available in the form of tablets in various dosages, including 0.25mg, 0.5mg, 1mg, 2mg, 3mg and 4mg for oral administration only.
Major depressive disorder (MDD) and schizophrenia
Major depression disorder (MDD) is a mental health condition characterised by frequent mood swings leading to feelings of sadness, loss, anger or frustration that are likely to interfere with daily life.
Though the exact reasons for depression are still unknown, it is believed that chemical changes in the brain are responsible for the disorder. It is estimated that approximately 15 million adults in the US live with MDD.
Schizophrenia is a chronic brain disabling disorder that causes people to hear imaginary voices, see imaginary things or believe other people are controlling their thoughts. In the US, approximately 2.4 million adults are suffering with schizophrenia.
Rexulti’s mechanism of action
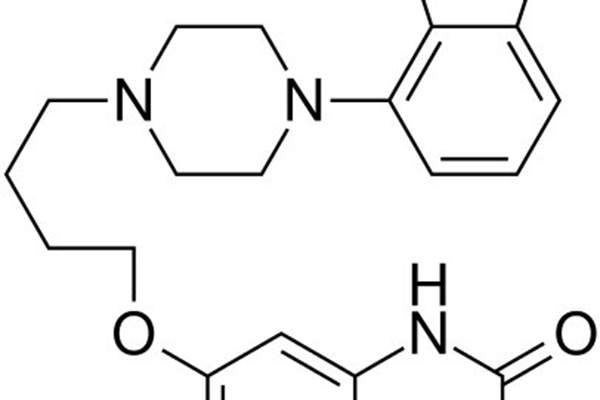
Rexulti contains an active ingredient called brexpiprazole, whose mechanism of action is not clearly known. It was proved the molecule achieves efficacy through a combination of partial agonist activity at serotonin 5-HT1A and dopamine D2 receptors, and antagonist activity at serotonin 5-HT2A receptors.
Clinical trials on Rexulti
Brintellix (vortioxetine) is a new multimodal antidepressant indicated for the treatment major depressive disorder (MDD) in adults.
The FDA approval of Rexulti was based on results from a clinical programme, which included four placebo-controlled, Phase III clinical studies. Two of the four studies were conducted on Rexulti as an adjunctive therapy to antidepressants in MDD and the remaining two in schizophrenia.
Rexulti’s efficacy as an adjunctive treatment for MDD was determined using two six-week, double-blind, placebo-controlled, fixed-dose trials conducted on adult patients who met the DSM-IV-TR criteria for MDD.
Patients in Study 1, which involved 228 participants, were randomised to either receive Rexulti 2mg or a placebo, administered once a day. Study 2 enrolled 227 patients, who were randomised to receive Rexulti 1mg or 3mg, or a placebo.
Patients randomised with Rexulti were initially treated with a 0.5mg dose of the drug during the first week followed by 1mg dose or increased to 2mg or 3mg from week three onwards. Dosages were then maintained for the remaining four weeks.
The primary endpoint for both studies was change in Montgomery-Åsberg Depression Rating Scale (MADRS).
Results showed that Rexulti at doses 2mg and 3mg were superior to a placebo, while the mean baseline MADRS score decreased from 27 by 8.36 (2mg) and 8.29 (3mg) compared to placebo reductions of 5.15 and 6.33.
A 1mg dose was not found superior to a placebo. The most common adverse reactions observed were akathisia and weight gain.
The efficacy of Rexulti as a treatment for schizophrenia in adults was determined in two, six-week, placebo-controlled, randomised, Phase III studies that compared fixed doses of the drug with a placebo.
Subjects were treated with an adequate dose of Rexulti for six weeks. The results demonstrated significant efficacy for the primary end point of Positive and Negative Syndrome Scale (PANSS).
In both studies, change from the baseline in PANSS total score for the drug was superior to a placebo. The most common adverse reaction observed during the studies was weight gain.