The product of a joint development programme between AstraZeneca and Bristol-Myers Squibb, saxagliptin is a member of a class of oral antidiabetic agents known as dipeptidyl peptidase IV (DPP-IV) inhibitors or "incretin enhancers".
In June 2008, Bristol-Myers Squibb and AstraZeneca submitted a new drug application to the US Food and Drug Administration (FDA) and a marketing authorisation application to the European Medicines Agency for saxagliptin.
The FDA approved the drug for the treatment of type 2 diabetes in August 2009. The drug is being marketed under the brand name Onglyza. In October 2009, saxagliptin was granted marketing authorisation from the European Union in 27 countries. To date saxagliptin has been approved in more than 66 countries including Canada, Mexico, China, Brazil and India.
In March 2010, Bristol-Myers Squibb and AstraZeneca initiated a Phase IV study to assess the safety and tolerability of saxagliptin in adult type 2 diabetes patients with cardiovascular risk factors. The five-year, multi-centre, randomised and double-blind study will track 12,000 diabetes patients.
AstraZeneca is currently conducting a series of Phase IIIb and IV studies on the drug. In June 2010, results from a 76-week Phase IIIb study in patients with type 2 diabetes with inadequate glycemic control were announced. The study, which evaluated saxaglitpin in combination with metformin, indicated that saxaglitpin provided long-term glycemic improvement in type 2 diabetes patients.
Incretins as a target for anti-diabetic medications
In the search for new anti-diabetic medications, the incretin system has emerged as an important target for new glucose-lowering drugs. Incretins, such as glucagon-like peptide-1 (GLP-1) and gastric inhibitor peptide, are naturally occurring hormones that are released from cells in the gut in response to food. They bind to receptors on pancreatic beta cells, stimulating the release of the hormone insulin, responsible for the regulation of blood sugar levels.
GLP-1 also reduces the secretion of glucagon, a hormone produced by the pancreas that stimulates the liver to convert glycogen to glucose, thus increasing blood sugar levels.
In type 2 diabetes, incretin function is impaired and patients are unable to properly regulate their blood-sugar levels. Saxagliptin inhibits DPP-IV, an enzyme responsible for the breakdown of GLP-1. By delaying GLP-1 degradation, saxagliptin extends the action of insulin while also suppressing the release of glucagon. This leads to a reduction in elevated blood glucose levels that characterises type 2 diabetes.
Saxagliptin effective for type 2 diabetes
The phase III trial programme for saxagliptin included studies in which it was administered as monotherapy as well as in combination with other standard antidiabetic drugs such as metformin, sulphonylureas, and thiazolidinediones.
In a 24-week randomised, multicentre, double-blind, placebo-controlled study in treatment naive type 2 diabetic patients, treatment with saxagliptin produced significant reductions in levels of glycosylated haemoglobin (HbA1C), fasting plasma glucose and postprandial glucose.
At once-daily doses of 2.5mg, 5mg and 10mg, the placebo-adjusted reductions in HbA1C were -0.6, -0.6 and -0.7% respectively (P<0.0001). At the highest dose, over 40% of patients achieved an HbA1C of less than 7% compared with 24% of placebo recipients (p<0.05).
Treatment with saxagliptin appeared well tolerated, with a side-effect profile similar to placebo. Encouragingly, there were no cases of confirmed hypoglycaemia in the placebo-controlled trial or any evidence of significant weight gain.
AstraZeneca is currently conducting a series of Phase IIIb and IV studies on the drug. In May 2010, a Phase IV study was initiated to assess the safety and tolerability of saxagliptin in adult type 2 diabetes patients with cardiovascular risk factors. The five-year, multi-centre, randomised and double-blind study will track 16,500 diabetes patients. It is expected to be completed by April 2014.
In June 2010, results from a 76-week Phase IIIb study in patients with type 2 diabetes with inadequate glycemic control were announced. The study, which evaluated saxagliptin in combination with metformin, indicated that saxagliptin provided long-term glycemic improvement in type 2 diabetes patients.
In September 2011, Astrazeneca and Bristol Myers Squibb announced results from a Phase IIIb multi-centre, randomised, placebo-controlled, double-blind study. The study enrolled 455 type 2 diabetic patients who were administered saxagliptin with insulin (with or without metformin). The primary end point was met and reduced HbA1c was observed.
In June 2011, a Phase III trial was initiated in 136 type 2 diabetic patients. The trial is being conducted to evaluate the safety and efficacy of the drug in paediatric patients aged between 10 and 17 years. The primary endpoint is to achieve reduced levels in HbA1c. The trial is expected to be completed in October 2014.
Another Phase III trial will be conducted to evaluate the safety and efficacy of the drug in combination with metformin. The trial will enrol 224 paediatric patients aged 10-17 years. It will be initiated in October 2011 and is scheduled for completion in April 2015.
Partnership in diabetes research
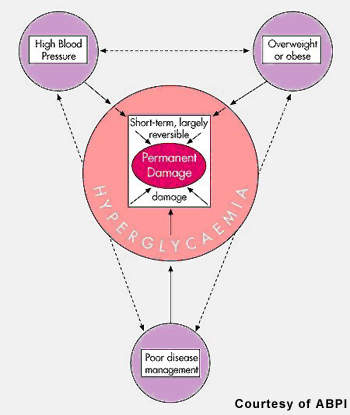
Estimates from the World Health Organization suggest that some 221 million people have diabetes, of whom more than 18 million live in the US. Type 2 diabetes accounts for 90-95% of all cases of diabetes. Worldwide the costs associated with treating the condition and its complications are estimated to exceed $200bn a year.
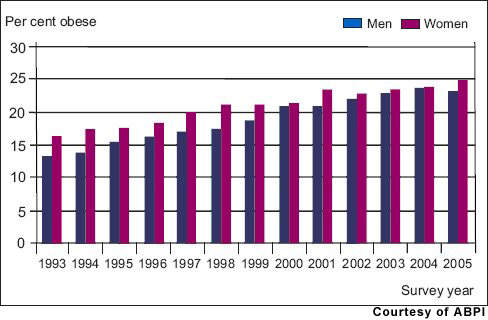
By 2030, the prevalence of diabetes is predicted to double, driven by adverse lifestyle changes that have seen an explosion in the incidence of obesity, a major risk factor for type 2 diabetes. Worldwide diabetes is a huge and growing problem, and for which new treatments are needed.
As part of their collaborative venture into the development of new anti-diabetic medications that was formed in 2007, AstraZeneca and Bristol-Myers Squibb are working on two investigational antidiabetic agents: saxagliptin and dapagliflozin.
While saxagliptin targets the incretin system, dapagliflozin (BMS-512148) is designed to inhibit the sodium-glucose uptake transporter (SGLT) in the proximal renal tubule of the kidneys. It is the first in this new class of anti-diabetic medications (called selective SGLT inhibitors) to enter clinical trials.
Marketing commentary
DPP-IV inhibitors are one of several new classes of antidiabetic medications in development for type 2 diabetes. Two, vildagliptin (Galvusâ) and sitagliptin (Januviaâ), are already approved and in clinical use.
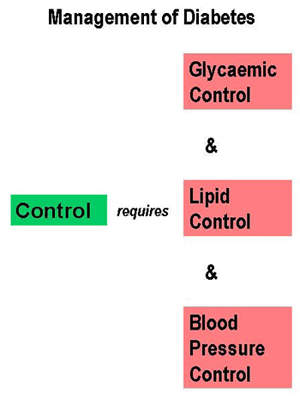
The ability of these agents to achieve sustainable reductions in HbA1c, the primary measure of blood glucose control, with an orally administered, well tolerated agent is seen as a major advantage over many older oral anti-diabetic drugs.