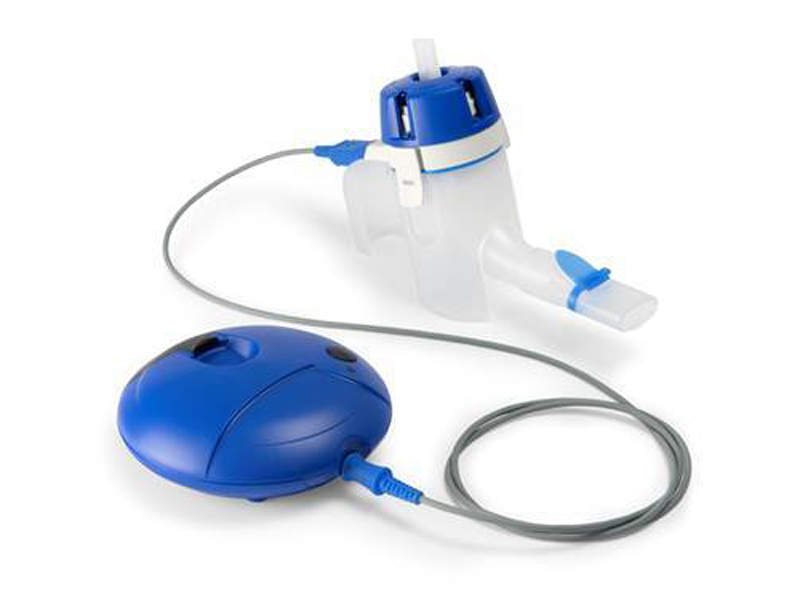
SUN-101 (Glycopyrrolate) is an inhalation solution developed by Sunovion Pharmaceuticals as a treatment for patients with moderate to severe chronic obstructive pulmonary disease (COPD).
The company has paired the drug with a closed eFlow nebuliser system developed by PARI Pharma. This delivers the drug to the target region in two to three minutes.
A new drug application (NDA) for SUN-101/eFlow was submitted to the US Food and Drug Administration (FDA) on 29 July 2016.
Chronic obstructive pulmonary disease
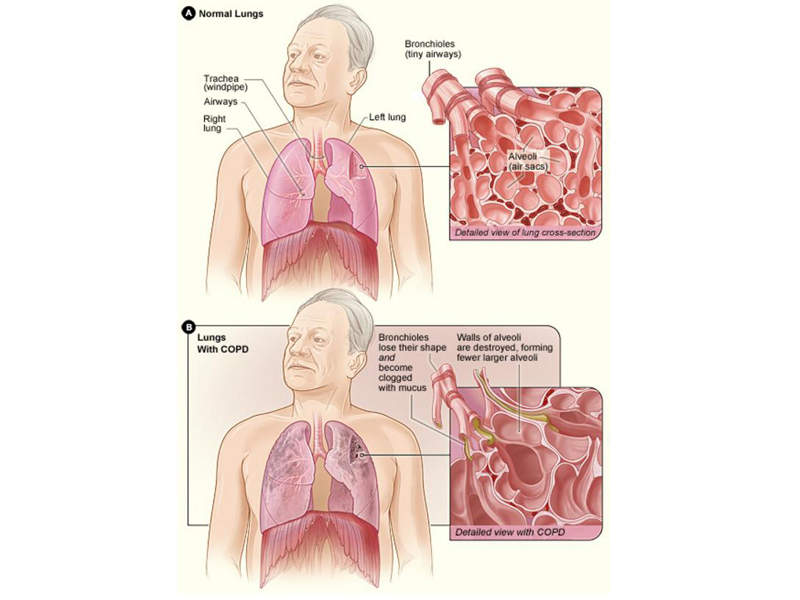
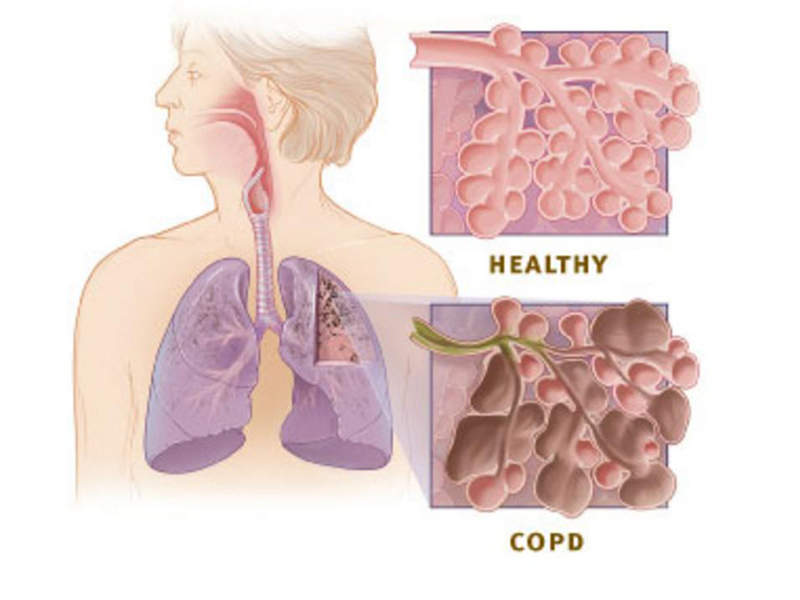
COPD is a lung disease, which includes chronic bronchitis, emphysema, and chronic airflow obstruction.
The disease is associated with symptoms such as increasing breathlessness, persistent cough, frequent lung infection, shortness of breath, and wheezing.
COPD may be caused by cigarette smoking, second hand smoke, air pollution, chemical fumes, and dust.
SUN-101’s mechanism of action
SUN-101 (glycopyrrolate) is a long-acting muscarinic antagonist (LAMA) bronchodilator, which binds to the muscarinic acetylcholine receptor and inhibits the action of acetylcholine to effectively perform dilation of the bronchi.
Clinical trials
A new drug application (NDA) for SUN-101/eFlow was submitted to the US FDA based on the results obtained from the GOLDEN-3, GOLDEN-4 and GOLDEN-5 trials.
GOLDEN-3 was a randomised, double-blind, placebo-controlled, multi-centre clinical trial conducted to evaluate the efficacy and the safety of the drug. It enrolled 653 COPD patients, who were randomised to receive SUN-101 50mcg or SUN-101 25mcg using an investigational eFlow closed system nebuliser or placebo twice a day for 12 weeks.
GOLDEN-4 was also a randomised, double-blind, placebo-controlled, parallel group, multi-centre trial conducted to evaluate the safety and efficacy of the drug. This triall enrolled 641 COPD patients, who were randomised to receive either SUN-101 or placebo twice daily for 12 weeks.
GOLDEN-3 and GOLDEN-4 studies have met the primary endpoints of change from baseline in trough Forced Expiratory Volume in 1 second (FEV1) at 12 weeks and secondary endpoint of change from baseline at 12 weeks in FEV1, change from baseline in trough forced vital capacity (FVC) at 12 weeks and change from baseline in health status.
GOLDEN-5 was a 48-week randomised, open-label, active-controlled, parallel group, multi-centre, long-term safety, and efficacy trial conducted on 1,087 COPD patients. Patients were randomised to receive either SUN-101 50mcg using eFlow delivery system twice a day or spiriva ( tiotropium bromide) 18mcg once a day using HandiHaler device.
The safety SUN-101 was assessed based on the number of treatment emergent adverse events (TEAE), serious adverse events (SAE) or major adverse cardiac events (MACE) and the number and percentage of study participants who discontinued the study due to TEAE.