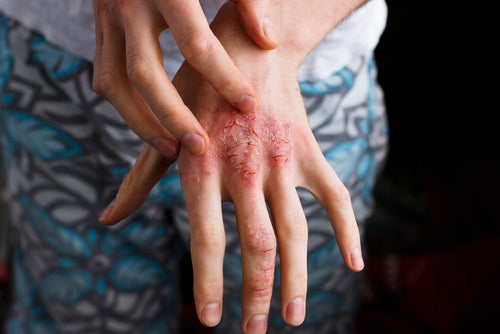
Alphyn Biologics has released new data from the first cohort of its Phase 2a clinical trial of AB-101a, a novel topical candidate to treat atopic dermatitis (AD). The data was presented in a poster at the World Congress of Dermatology in Singapore.
Atopic dermatitis is the most common form of dermatitis, causing the skin to become dry, cracked, and itchy. It is more common in children than adults and causes often relate to a gene variation affecting the skin’s ability to provide protection or excessive bacteria Staphylococcus aureus on the skin displacing helpful bacteria. Both causes disrupt the skin’s protective barrier.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The randomised, double-blind, vehicle-controlled trial included 41 patients over four weeks and seven sites to assess the safety and efficacy of the topical hydrogel AB-101a. The randomisation resulted in the enrolment of 81% of participants with mild AD. The trial met all primary and secondary endpoints with few reported safety issues or side effects.
The Phase 2a trial assessed statistically significant improvement in the Investigator Global Assessment (IGA) score, which is a five-point scale providing a clinical assessment of AD severity ranging from 0 to 4, where 0 indicates clear, 2 is mild, 3 is moderate, and 4 indicates severe AD. The IGA was reported as reaching clear or almost clear within four weeks.
AB-101a achieved statistical significance for improvement in Eczema Area and Severity Index (EASI) score, including reaching EASI 75 score, meaning there was an equal to or greater than 75% improvement from baseline. Furthermore, the body surface area of AD declined by at least 50%.
There was a significant improvement throughout the trial in the Skin Infection Rating Scale (SIRS), which demonstrates control of the bacterial microbiome on the AD skin and improves the ability of the immune system to manage flare-ups and prevent infection.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe itch score improvement was equal to or greater than 4 in the paediatric group. This continuously increased and was sustained after the dosing stopped.
US-based Alphyn is conducting a second Phase 2a trial for AB-101a, with the second cohort of the trial in an open-label study to evaluate patients with mild, moderate, and severe AD with secondary infection and control of the bacterial microbiome on the AD skin. The 20-patient trial has completed enrolment and interim results from the second cohort will be presented in a poster at the Society for Paediatric Dermatology annual meeting from 13 to 16 July.
GlobalData estimates that the AD market is expected to grow from $6.4bn in 2020 to $16.8bn in 2030 in the seven major markets (United States, EU5 (Germany, France, Italy, Spain, and the United Kingdom) and Japan) at a compound annual growth rate of 10.1%.





