Amylyx Pharmaceuticals has announced positive topline results from the Phase II HELIOS clinical trial, indicating sustained improvements with AMX0035 in adults with Wolfram syndrome.
AMX0035 is an oral combination therapy designed to slow or mitigate neurodegeneration by acting on endoplasmic reticulum (ER) stress and mitochondrial dysfunction. It is being studied as a potential therapy for the syndrome and progressive supranuclear palsy.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
HELIOS is a single-arm, single-site, open-label Phase II trial designed to study AMX0035’s effects on safety, tolerability and various functions in adult participants.
It includes up to 96 weeks of treatment followed by a four-week safety follow-up.
Amylyx chief medical officer Camille Bedrosian said: “AMX0035 is believed to target both of these critical pathways. In addition, we are encouraged by the sustained improvement observed in all participants who completed Week 36 or Week 48 assessments, and we thank the Wolfram syndrome community for their continued collaboration and support in researching the potential of AMX0035.
“We continue to engage with stakeholders and plan to meet with the FDA to inform a Phase III programme.”

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
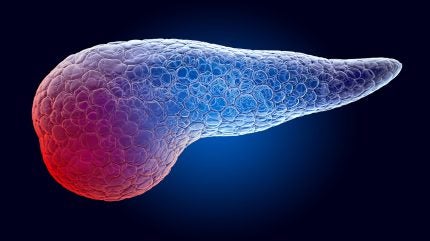
By GlobalDataThe HELIOS trial showed that AMX0035 led to improvements in pancreatic function, as measured by C-peptide response, in contrast to the expected decrease due to disease progression.
Longer-term data from participants who completed week 36 and week 48 assessments revealed sustained improvement over time.
The trial’s primary efficacy endpoint, C-peptide response, showed a positive change from baseline at week 24. Secondary and exploratory outcomes assessed other diabetic measures and domains affected by Wolfram syndrome.
Secondary endpoints, including hemoglobin A1c (HbA1c) levels, glucose range and visual acuity, also showed improvements or stabilisation.
Participants receiving AMX0035 experienced improved glycemic control, visual acuity and disease stabilisation or improvement, as reported by both clinicians and patients.
The US Food and Drug Administration and the European Commission have granted orphan drug designation to AMX0035.
In July this year, Amylyx announced plans to rebuild its pipeline by discontinuing Relyvrio and acquiring Phase III-ready GLP-1 receptor antagonist avexitide from Eiger BioPharmaceuticals for $35.1m.





