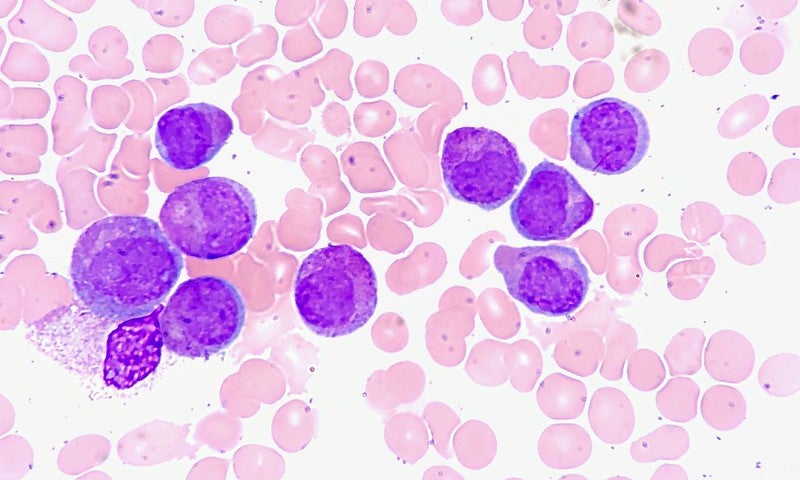
Aptose Biosciences has commenced dosing of participants in the Phase I/II APTIVATE clinical trial of tuspetinib (formerly HM43239) in acute myeloid leukemia (AML) patients.
The dose escalation, open-label, multicentre, and expansion Phase I/II APTIVATE study has been designed for evaluating the pharmacodynamics, tolerability, safety, and pharmacokinetics of HM43239 in relapsed or refractory (R/R) AML patients.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
It will also confirm HM43239’s monotherapy activity through patient enrichment of specific, mutationally defined AML populations, including FLT3-mutant patients and TP53-mutant patients who were failed by a previous FLT3 inhibitor.
The APTIVATE expansion trial will also assess the PK parameters, tolerability, and safety of HM43239, along with venetoclax, in R/R AML patients.
The once daily oral agent tuspetinib has been designed to target SYK, JAK1/2, FLT3, and other kinases operative in AML, simultaneously.
As a monotherapy, tuspetinib was found to safely deliver several complete remissions and clinical responses across 40mg, 80mg, 120mg, and 160mg dose levels in AML patients.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThese patients were previously failed by chemotherapy, FLT3 inhibitors, BCL2 inhibitors, hematopoietic stem cell transplants, and hypomethylating agents.
Aptose Biosciences chairman, president, and CEO William Rice said: “We are pleased to have dosing underway in our APTIVATE clinical trial of tuspetinib in a very ill R/R AML population.
“Tuspetinib has demonstrated noteworthy safety and mutation agnostic potency across a spectrum of AML patients with a diversity of adverse mutations, further distinguishing it from competing compounds and targeting a much larger AML population.
“This breadth of activity, along with its significant safety profile, has allowed us to define a precise clinical and commercial plan for tuspetinib in multiple lines of therapy, including its use in doublet and triplet combinations, as well as maintenance therapy.”
The company also stated that an R/R AML patient receiving 40mg tuspetinib orally once a day in the dose exploration trial has achieved another clinical response.





