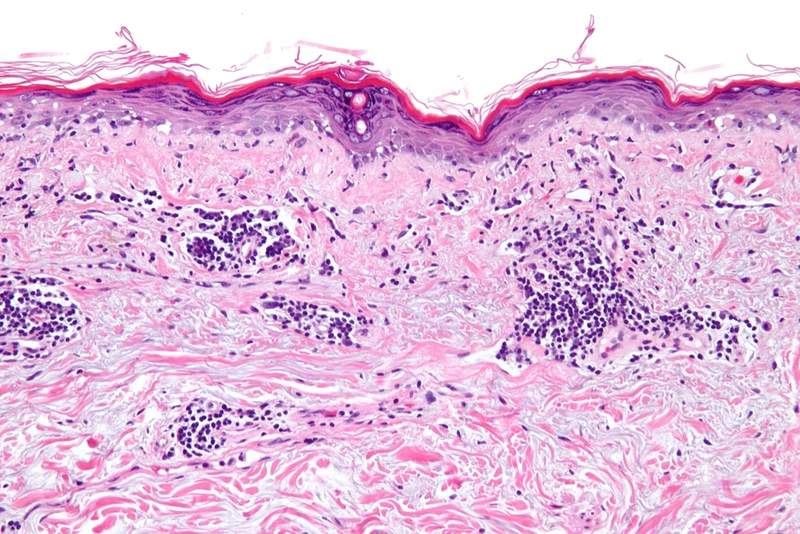
AstraZeneca has reported negative top-line results from the TULIP 1 trial after the study failed to meet its primary endpoint.
The trial is part of the pivotal Treatment of Uncontrolled Lupus via the Interferon Pathway (TULIP) that comprises two Phase III clinical trials, TULIP 1 and TULIP 2.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
TULIP 1 was a randomised, double-blinded, 52-week placebo-controlled, multi-centre study that investigated the safety and efficacy of anifrolumab for the treatment of adult patients with moderate-to-severe systemic lupus erythematosus (SLE).
As part of the trial, 460 patients were enrolled and were randomised in 1:2:2 ratio to get a fixed-dose intravenous infusion of 150mg of anifrolumab, 300mg of anifrolumab, or placebo every four weeks.
The primary endpoint of the trial was achieving a statistically significant reduction in disease activity in the enrolled patients as measured by the SLE Responder Index 4 (SRI4) at 12 months.
The overall TULIP programme is also designed to evaluate the decreasing use of oral corticosteroids, enhancing skin manifestations, as measured by Cutaneous Lupus Erythematosus Disease Area and Severity Index (CLASI), as well as minimising flares.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataAstraZeneca Global Medicines Development executive vice-president and chief medical officer Sean Bohen said: “SLE is a debilitating autoimmune disease with significant unmet need among patients who struggle to achieve meaningful disease control.
“The result of this trial is disappointing for patients and the lupus community.”
In addition, AstraZeneca is currently evaluating anifrolumab in a Phase III SLE long-term extension trial, a Phase II trial using subcutaneous delivery in SLE, as well as a Phase II trial for lupus nephritis.
SLE is an autoimmune disease where the immune system attacks healthy tissue in the body rather than primarily targeting viruses or other foreign invaders.



