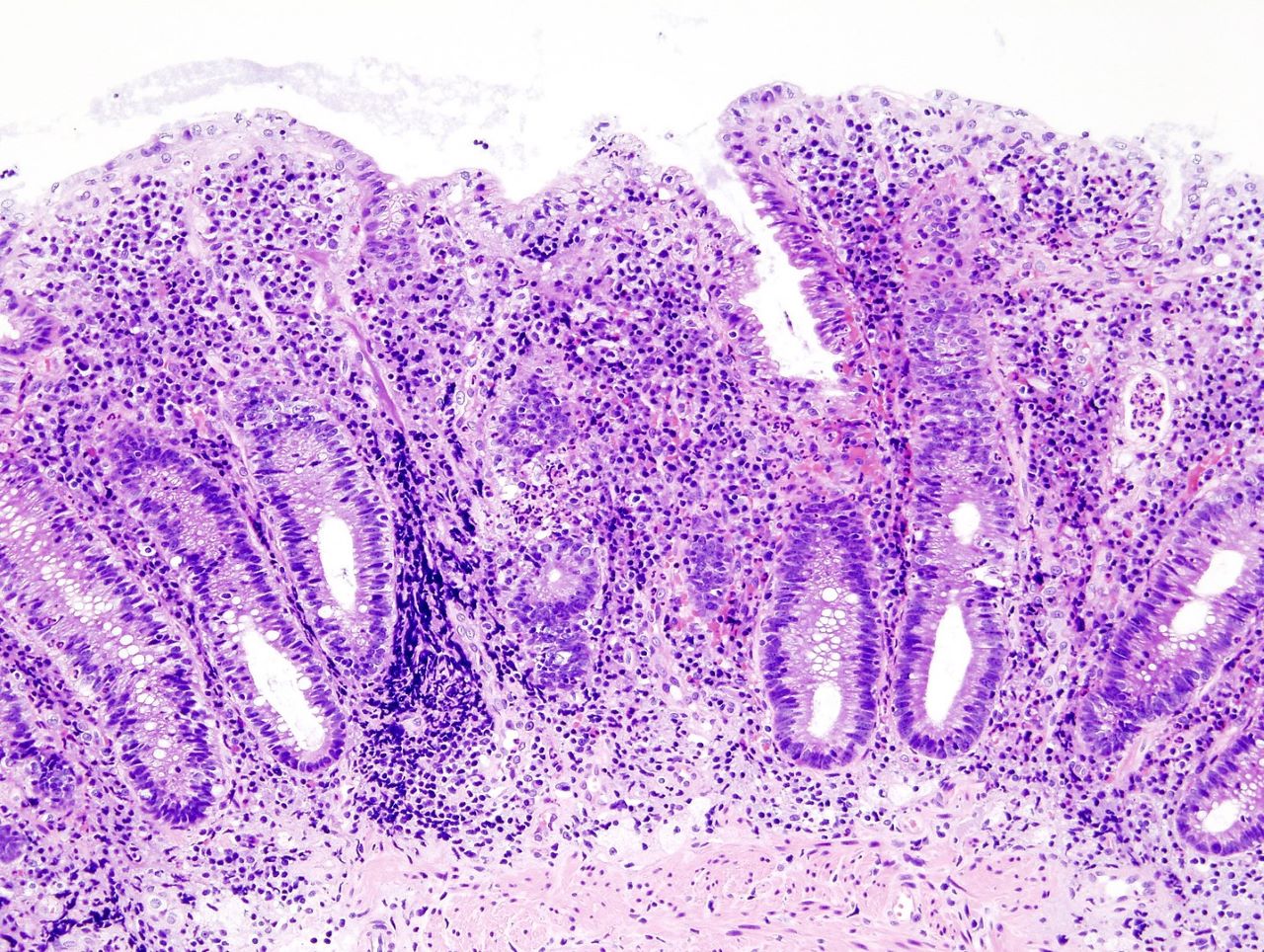
Bacainn Therapeutics has reported positive results from the Phase I clinical trial of gut-restricted drug, BT051, for treating inflammatory diseases such as ulcerative colitis.
The company announced the successful completion of the first-in-human trial after the drug was observed to be safe and well-tolerated.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
BT051 is an orally administered drug designed to target neutrophil migration and subsequent activation.
Neutrophils migrate into the submucosa and lumen of the colon during active flares of ulcerative colitis.
It can lead to tissue damage due to the release of a cocktail of destructive enzymes, DNA, histones and pro-inflammatory mediators.
The randomised, double-blind, placebo-controlled, single-centre trial was conducted on healthy participants who were randomised into five sequential, single-ascending-dose cohorts.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe arms received a 100mg, 300mg, 700mg, 1,500mg, and 3,500mg dose of BT051 or a placebo.
Trail results showed that single doses of BT051 up to 3,500mg were generally safe and well-tolerated in all subjects.
Bacainn Therapeutics chief medical officer Chris Stevens said: “The safety and tolerability results from this first-in-human trial of BT051 are encouraging as they support our efforts to develop BT051 as a treatment for inflammatory bowel disease.
“There is significant evidence to support the role of overactive neutrophils in the large intestine as a driver of inflammation in inflammatory bowel disease, and BT051 has the potential to be a first-in-class medicine.”
No serious adverse events related to the drug were observed in the trial.
In addition, undetectable or minimal systemic exposure and high stool concentrations were observed across all the doses.
Bacainn Therapeutics anticipates initiating a Phase Ib clinical trial of BT051 in patients with moderate to severe active ulcerative colitis next year.
Full data from the completed Phase I clinical trial will be shared soon.





