Swedish pharmaceutical company Camurus has reported positive results from the Phase III ACROINNOVA 2 study, which assessed the efficacy and safety of octreotide subcutaneous (SC) depot (CAM2029) in patients with acromegaly.
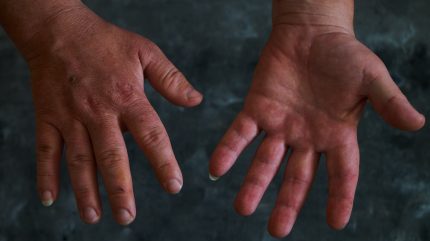
Acromegaly is a slowly progressive disease caused by a tumour of the pituitary gland producing excess growth hormone and stimulating increased insulin growth factor-1 (IGF-1) levels.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
This results in abnormal growth of bone and tissue, causing enlarged hands, feet, facial features and inner organs, as well as symptoms such as fatigue, joint pain, headache, visual field defects, excessive sweating and paresthaesia.
CAM2029 is an investigational, ready-to-use octreotide designed to treat acromegaly in addition to gastroentero-pancreatic neuroendocrine tumours and polycystic liver disease.
The drug is currently being reviewed by the US Food and Drug Administration (FDA) and European Medicines Agency (EMA), with the FDA expected to issue a first approval decision by 21 October.
The ACROINNOVA 2 trial’s primary focus was on safety over a year of treatment, with the drug demonstrating a favourable profile.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe study involved 135 patients, with 81 new participants and 54 from the previous ACROINNOVA 1 trial.
Its primary endpoint was CAM2029’s safety over 52 weeks, while secondary endpoints included biochemical control rates, symptom scores and patient-reported outcomes.
CAM2029 was found to be well-tolerated in the trial, with no new safety concerns compared to standard-of-care somatostatin receptor ligands.
Adverse events were mostly mild to moderate, with the most common being injection site reactions and gastrointestinal issues.
Camurus CSO president and CEO Fredrik Tiberg said: “Today’s results from ACROINNOVA 2 highlight the long-term safety profile and efficacy of octreotide SC depot in patients with acromegaly, including patients with uncontrolled disease on standard-of-care.
“These data further strengthen the evidence base for CAM2029 octreotide SC depot as a new treatment option for people living with acromegaly, if approved.”





